【Corning】354262 产品使用方法
Corning® Matrigel® Matrix High Concentration (HC), Phenol-Red Free, LDEV-free
(354262)
基底膜是体内细胞下方的薄层细胞外基质。Corning Matrigel Matrix High Concentration (HC) Phenol Red Free是从Engelbreth-Holm-Swarm(EHS)小鼠肉瘤中提取的可溶化基底膜制备物,该肿瘤富含细胞外基质蛋白。其主要成分是层粘连蛋白,其次是胶原蛋白IV、肝素硫酸酯蛋白多糖和接触素/巢蛋白。Corning Matrigel Matrix HC Phenol Red Free还含有TGF-β、表皮生长因子、胰岛素样生长因子、成纤维细胞生长因子、组织纤溶酶原激活剂等生长因子,这些生长因子在EHS肿瘤中自然存在。Corning Matrigel Matrix HC Phenol Red Free可有效促进正常和转化的依赖锚定的上皮细胞和其他细胞类型的附着和分化,包括神经元、肝细胞、Sertoli细胞、小鸡晶状体和血管内皮细胞。Corning Matrigel Matrix HC Phenol Red Free会影响成年大鼠肝细胞、血管内皮细胞以及小鼠和人乳腺上皮细胞的三维培养中基因表达。它是几种肿瘤细胞侵袭实验的基础,支持体内周围神经再生,并为体外和体内研究血管生成提供必要的基质。Corning Matrigel Matrix HC Phenol Red Free还支持免疫抑制小鼠体内人类肿瘤的繁殖。还可以用于移植未经分选的乳腺细胞,以及嵌入在Corning Matrigel Matrix中的分选的上皮亚群。这种基质也被用作癌症干细胞模型,并且研究表明可以增强肿瘤在体内的生长速度。
应用:
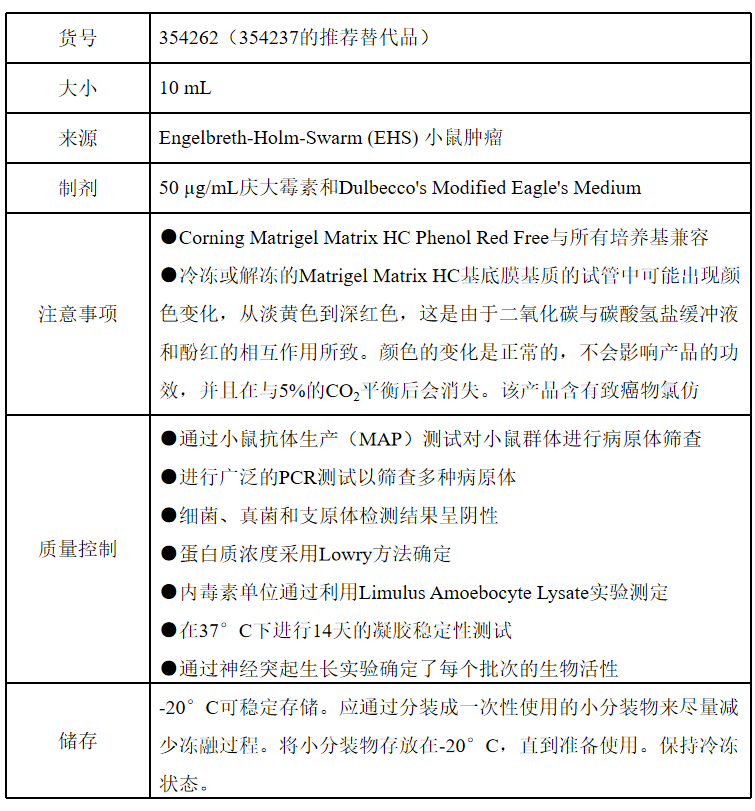
表 1 354262产品信息
溶解方法
与Corning Matrigel Matrix HC Phenol Red Free接触的所有培养器具或培养基都必须预先冷却/冰冷,因为Corning Matrigel Matrix HC Phenol Red Free在10°C以上会开始凝胶化。始终将Corning Matrigel Matrix保持在冰上。
将Corning Matrigel Matrix HC Phenol Red Free解冻的方法是将小瓶完全浸入冰中并放入4°C冰箱的后部过夜。一旦Corning Matrigel Matrix HC Phenol Red Free解冻,摇晃瓶子以确保物质均匀分散。始终将Corning Matrigel Matrix放在冰上。采用无菌技术处理。将解冻的Corning Matrigel Matrix HC Phenol Red Free小瓶放入无菌区域,喷洒70%乙醇于瓶盖上,并风干。
可以使用预冷的移液管轻轻吸取Corning Matrigel Matrix HC Phenol Red Free,以确保均匀性。将Corning Matrigel Matrix HC Phenol Red Free分装到管中,当Corning Matrigel Matrix HC Phenol Red Free堵塞移液枪头端并/或导致移液管测量不准确时,请更换吸头。
如果凝胶化的Corning Matrigel Matrix HC Phenol Red Free放置在4°C的冰中24-48小时,可以重新液化。
Corning Matrigel Matrix HC Phenol Red Free可以作为薄凝胶层(0.5 mm),在其上培养细胞。也可以在Corning Matrigel Matrix HC Phenol Red Free内培养细胞,使用1 mm的层厚。过度稀释会导致薄而非凝胶化的蛋白质层。这对于细胞附着可能是有用的,但在分化研究中可能不太有效。
注射方案
Corning Matrigel Matrix HC Phenol Red Free可以通过皮下注射到小鼠体内(Corning Matrigel Plug Assay)来评估不同化合物的体内血管生成活性。高蛋白浓度促进肿瘤的生长,并使Corning Matrigel Plug在注射后保持完整性。这使得注入的肿瘤和/或血管生成化合物局限于原位分析和/或未来切除。
注意:Corning Matrigel Matrix产品的蛋白质浓度是批次特异性的,并在分析证书上提供。为了获得一致的结果,通过计算所需的特定蛋白质浓度(mg/mL)来稀释Corning Matrigel Matrix产品。为了保持凝胶的一致性,建议不要将Corning Matrigel Matrix稀释至低于3 mg/mL。使用冰冷的无血清培养基来稀释Corning Matrigel Matrix。冰冷的培养基可以直接加入冷冻的Corning Matrigel Matrix HC Phenol Red Free小瓶中,并按照建议的方法进行解冻。通过上下移液或在冰中旋转小瓶混合。
在将Corning Matrigel Matrix HC Phenol Red Free和Corning Matrigel Matrix/细胞悬浮液注射到小鼠之前,保持它们尽可能冷却,但不要冷冻。在整个过程中,保持Corning Matrigel和Corning Matrigel Matrix/细胞悬浮液尽可能无菌非常重要。
● 对于每只接受注射的小鼠,在冰上将细胞(2x105个或更多)和Corning Matrigel Matrix HC Phenol Red Free混合在最终体积为0.5 mL的溶液中。
●细胞应尽可能以较小的体积存在。通常情况下,将250 µl冰冷的含有2x106cells/mL的培养基与250µl冰冷的Corning Matrigel Matrix HC Phenol Red Free混合。
●使用19G针(对于组织样本)或23G针(对于培养细胞)在裸鼠的皮下注射细胞。注射应迅速进行,以防止Corning Matrigel Matrix HC Phenol Red Free凝固。
参考文献
[1]. Kleinman HK, et al, Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma, Biochemistry 21:6188 (1982).
[2]. Klenman HK, et al, Basement membrane complexes with biological activity, Biochemistry 25:312 (1986).
[3]. Vukicevic S, et al, Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular activity related to extracellular matrix components, Exp Cell Res 202:1 (1992).
[4]. McGuire PG and Seeds NW, The interaction of plasminogen activator with a reconstituted basement membrane matrix and extracellular macromolecules produced by cultured epithelial cells, J. Cell. Biochem. 40:215 (1989).
[5]. Biederer T and Scheiffele P, Mixed-culture assays for analyzing neuronal synapse formation, Nat Protoc 2(3):670 (2007).
[6]. Li Y, et al, Essential Role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor, Nature 434:894 (2005).
[7]. Bi Y, et al, Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport, Drug Metab Dispos. 34(9):1658 (2006).
[8]. Gassei K, et al, Immature rat seminiferous tubules reconstructed in vitro express markers of Sertoli cell maturation after xenografting into nude mouse hosts, Mol Hum Reprod. 16(2):97 (2010).
[9]. Yu X, et al, Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat sertoli cell/gonocyte co-cultures: An improved in vitro model for assessment of male reproductive toxicity, Toxicol Sci 84(2):378 (2005).
[10]. Chandrasekher G, and Sailaja D, Differential activation of phosphatidylinositol 3-kinase signaling during proliferation and differentiation of lens epithelial cells, Invest Ophthalmol Vis Sci. 44(10):4400 (2003).
[11]. McGuire PG, and Orkin RW, A simple procedure to culture and passage endothelial cells from large vessels of small animals, Biotechniques 5(6):456 (1987).
[12]. Bissel DM, et al, Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver, J. Clin Invest. 79:801 (1987).
[13]. Page JL, et al, Gene expression profiling of extracellular matrix as an effector of human hepatocyte phenotype in primary cell culture, Toxicol Sci 97(2):384 (2007).
[14]. Cooley LS, et al, Reversible transdifferentiation of blood vascular endothelial cells to a lymphatic-like phenotype in vitro, J Cell Sci. 123(Pt 21):3808 (2010).
[15] Li ML, et al, Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells, Proc. Nat. Acad. Sci. USA 84:136 (1987).
[16] Barcellof MH, et al, Functional differentiation and aveolar morphogenesis of primary mammary cultures on reconstituted basement membrane, Development 105:223 (1989).
[17]. Roskelley CD, et al, Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction, Proc. Nat. Acad. Sci. USA 91(26):12378 (1994).
[18]. Xu R, et al, Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors, J. Biol. Chem. 282(20):14992 (2007).
[19]. Debnath J, et al, Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures, Methods 30(3):256 (2003).
[20]. Muthuswamy SK, et al, ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini, Nat. Cell Biol. 3(9):785 (2001).
[21]. Albini A, et al, A rapid in vitro assay for quantitating the invasive potential of tumor cells, Cancer Res,47:3239 (1987).
[22]. Poincloux R, et al, Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel, Proc Natl Acad Sci USA.108(5):1943 (2011).
[23]. Madison R, et al, Increased rate of peripheral nerve regeneration using bioresorbable nerve guides and laminin containing gel, Exp. Neurology 88:767 (1985).
[24]. Xu XM, et al, Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord, J. Comp. Neurol. 351(1):145 (1994).
[25]. Lopatina T, et al, Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo, PLoS One 6(3):e17899 (2011).
[26]. Kubota Y, et al, Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures, J. Cell Biol. 107:1589 (1988).
[27] Ponce ML, Tube formation: an in vitro matrigel angiogenesis assay, Methods Mol Biol. 467:183 (2009).
[28] Passaniti A, et al, A simple, quantitative method for assessing angiogenesis and anti-angiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor, Lab Invest. 67:519 (1992).
[29] Isaji M, et al, Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo, Br J Pharmacol 122:1061 (1997).
[30] Adini A, et al, Matrigel cytometry: a novel method for quantifying angiogenesis in vivo, J Immunol Method. 342(1-2):78 (2009).
[31] Albini A, et al, Matrigel promotes retinoblastoma cell growth in vitro and in vivo, Int. J. Cancer 52(2):234 (1992).
[32] Yue W, and Brodie A, MCF-7 human breast carcinomas in nude mice as a model for evaluating aromatase inhibitors, J. Steroid Biochem. Mol. Biol. 44(4-6):671 (1993).
[33]. Angelucci A, et al, Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice, Endocr-Relat Cancer 13(1):197 (2006).
[34] Moraes RC, et al, Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia, Development 134:1231 (2007).
[35] Zeng YA, and Nusse R, Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture, Cell Stem Cell 6:568 (2010).
[36] Jeselsohn R, et al, Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV ErbB2 tumorigenesis, Cancer Cell 17:65 (2010).
[37] Quintana E, et al, Efficient tumor formation by single human melanoma cells, Nature 456:593 (2008).
[38]. Anai S, et al, Dual targeting of Bcl-2 and VEGF: a potential strategy to improve therapy for prostate cancer, Urol Oncol 29:421 (2011).
[39]. Cao J, et al, A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap, Invest Ophthalmol Vis Sci 51:6009 (2010).
其他细胞品类
别划走,精彩继续

长按识别
添加一对一专属技术支持

扫码进群
咨询详细产品信息

点在看,传递你的品味