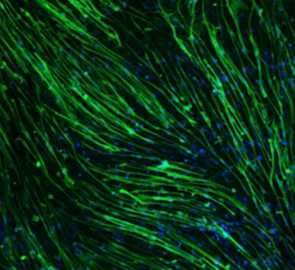
【Merck】使用永生化16HBE14o-人支气管上皮细胞系模拟呼吸性肺疾病
试剂耗材准备
货号 | 说明 |
SCC150 | 16HBE14o- Human Bronchial Epithelial Cell Line 16HBE14o- human bronchial epithelial cell line is widely used to model barrier function of the airway epithelium and to study respiratory ion transport as well as the function of CFTR. |
SCC152 | 1HAEo- Human Airway Epithelial Cell Line 1HAEo- human airway epithelial cell line is a useful model for the study of epithelial ion transport, secretion and biochemistry. |
SCC154 | 56FHTEo- Human Tracheal Epithelial Cell Line 56FHTEo- human tracheal epithelial cell line is a useful model for the study of epithelial ion transport, secretion and biochemistry. |
SCC157 | 6CFSMEo- Human Cystic Fibrosis Submucosal Gland Epithelial Cell Line 6CFSMEo- human CF submucosal gland epithelial cell line was derived from a cystic fibrosis patient who was compound heterozygote for the ΔF508 and Q2X CFTR mutations. |
126575 | Albumin, Bovine Serum, Fraction V, Fatty Acid-Free |
MAB3484 | Anti-Cystic Fibrosis Transmembrane Conductance Regulator Antibody, a.a. 386-412, clone L12B4 clone L12B4, Chemicon®, from mouse |
MAB3412 | Anti-Cytokeratin AE1/AE3 Antibody, recognizes acidic & basic cytokeratins, clone AE1/AE3clone AE1/AE3, Chemicon ®, from mouse |
AB2272 | 抗ZO -1抗体from rabbit, purified by affinity chromatography |
SCC159 | CFBE41o- 4.7 DeltaF508-CFTR Human CF Bronchial Epithelial Cell Line CFBE41o- 4.7 ΔF508-CFTR human CF bronchial epithelial cell line may be used to study the relationship between CFTR mRNA expression and Cl transport function. |
SCC158 | CFBE41o- 4.7 WT-CFTR Human CF Bronchial Epithelial Cell Line CFBE41o- 4.7 WT-CFTR human CF bronchial epithelial cell line may be used to study the relationship between CFTR mRNA expression and Cl transport function. |
SCC161 | CFBE41o- 6.2 DeltaF508-CFTR Human CF Bronchial Epithelial Cell Line CFBE41o- 6.2 ΔF508-CFTR human CF bronchial epithelial cell line may be used to study the relationship between CFTR mRNA expression and Cl transport function. |
SCC160 | CFBE41o- 6.2 WT-CFTR Human CF Bronchial Epithelial Cell Line CFBE41o- 6.2 WT-CFTR human CF bronchial epithelial cell line is used to study the relationship between CFTR mRNA expression and Cl transport function. |
SCC151 | CFBE41o- Human CF Bronchial Epithelial Cell Line CFBE41o- human CF bronchial epithelial cell line was derived from a cystic fibrosis patient homozygous for the ΔF508 CFTR mutation. |
SCC155 | CFSMEo- Human Cystic Fibrosis Submucosal Gland Epithelial Cell Line CFSMEo- human CF submucosal gland epithelial cell line was derived from a cystic fibrosis patient who was compound heterozygote for the ΔF508 and Q2X CFTR mutations. |
SCC162 | CFTE29o- Human Cystic Fibrosis Tracheal Epithelial Cell Line CFTE29o- human cystic fibrosis tracheal epithelial cell line is a useful model for cystic fibrosis research and for studying chloride ion transport and ion transport in human airways. |
MERS00002 | Millicell ERS-2 电压电阻表 The Millicell-ERS (Electrical Resistance System) reliably measures membrane potential & resistance of epithelial cells in culture. |
MCHT12H48 | MCHT12H48 Millicell® Hanging Cell Culture Insert, PET 0.4 μm, 12-well, 48/pk Polyethylene Terephthalate hanging cell culture insert with pore size of 0.4 μm used in a 12-well plate for cell attachment, cell culture, cell differentiation & ICC. |
实验步骤
一、气液界面(Air-Liquid Interface)3D培养
气液界面(ALI)3D细胞培养技术支持将人支气管上皮细胞从16HBE14o-等细胞分化为成熟的肺表型。使用ALI细胞培养技术培养的支气管上皮细胞可以形成极化细胞层和紧密连接,并且可以分化和呈现功能性纤毛。ALI细胞培养方法依赖于Millicell插入式细胞培养小室来支持体内观察到的假复层粘液纤毛表型的发展。
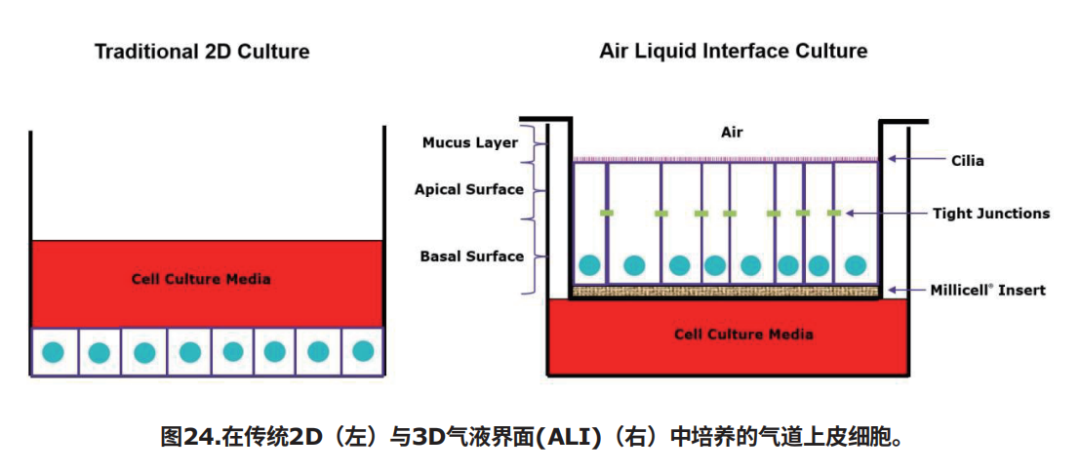
图1.在传统2D(左)与3D气液界面(ALI)(右)中培养的气道上皮细胞。
气液界面细胞培养方案
1. 用ECM混合物涂抹组织培养瓶:
• 纤连蛋白 (FN) (10 μg/mL,F2006)
• 胶原蛋白(30 μg/mL,5006)
• BSA (100 μg/mL,126575) 在含有α-MEM(M2279)、 10% FBS (ES-009-B)、2mM L-谷氨酰胺(TMS-002-C)和1×青霉素-链霉素溶液(TMS-AB2-C)的ECM涂层烧瓶中培养和扩增 16HBE14o-细胞。不要让细胞在达到90-95%汇合后过度汇合和分裂。用胰蛋白酶-EDTA溶液(T3924)去除贴壁细胞。
2. 将16HBE14o-细胞接种以105个细胞/cm2 的密度接种到Millicell细胞培养小室 (MCHT12H48)中,将细胞浸入上述步骤1中列出的1-2mL扩增培养基中。将细胞在37°C的培养箱中培养24小时。
3. 启动气液界面培养,将培养小室/细胞转移到新的深12孔板(Greiner Bio-One ThinCert板)中。可以去除小室中的整个培养基,使细胞暴露在空气中。每2天更换一次小室背面外侧的培养基。
4. 在第8天或之后,可以使用常见的基于细胞的测定或抗体染色来分析细胞。
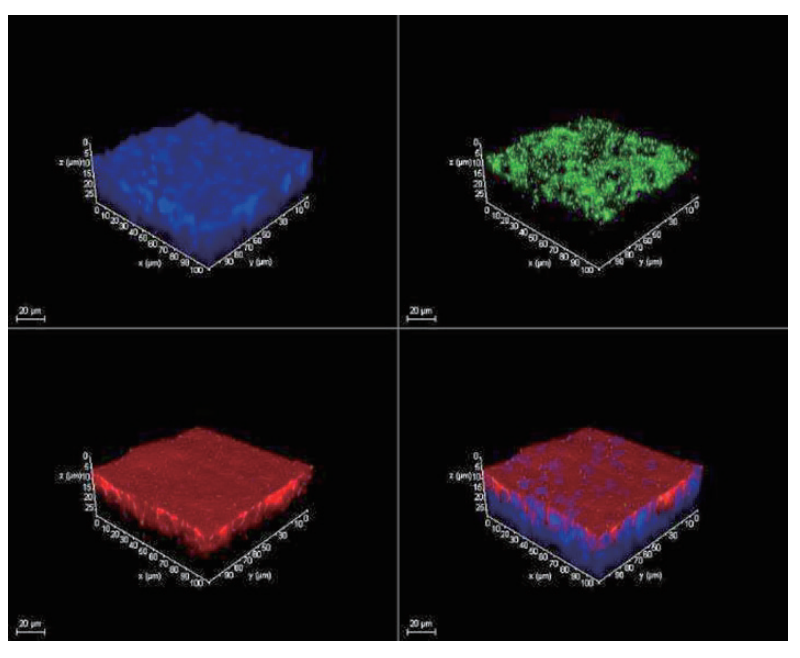
图2.人支气管上皮细胞人支气管上皮细胞的ALI培养。人支气管上皮细胞细胞在气-液界面( ALI ) (红蓝色)培养时呈现极性(蓝色)、细胞内紧密连接ZO-1 ( 红色 , AB2272 )和顶端CFTR表达( 绿色 , MAB3484 ),具有上皮屏障功能。
二、囊性纤维化细胞模型和CFTR功能
囊性纤维化是一种遗传性疾病,影响包括肺和消化系统等多个器官系统。这种疾病的特征是黏液分泌腺体异常,导致分泌物扰乱气道,粘稠的黏液堵塞肺部,可能导致死亡。囊性纤维化跨膜电导调节剂 (CFTR)蛋白有助于维持体内包括肺表面的盐水平衡。CFTR 基因的突变或缺失可导致囊性纤维化。
16HBE14o-细胞系 (SCC150) 是一种野生型气道上皮细胞系,可表达高水平的CFTR mRNA和蛋白质。亲本 CFBE41o-细胞系(SCC151)源自CF患者的ΔF508 CFTR突变纯合子。已创建该系的后续克隆,并校正了ΔF508 CFTR突变 (SCC158-SCC161)。
16HBE14o-囊性纤维化参考文献
1. Andersson C, Al-Turkmani MR, Savaille JE, Alturkmani R, Katrangi W, et al. 2008. Cell culture models demonstrate that CFTR dysfunction leads to defective fatty acid composition and metabolism. J Lipid Res. 49(8):1692-700
2. Muir A, Soong G, Sokol S, Reddy B, Gomez MI, et al. 2004. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 30(6):777-83.
3. Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, et al. 2010. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROSmediated autophagy inhibition. Nat Cell Biol. 12(9):863-75.
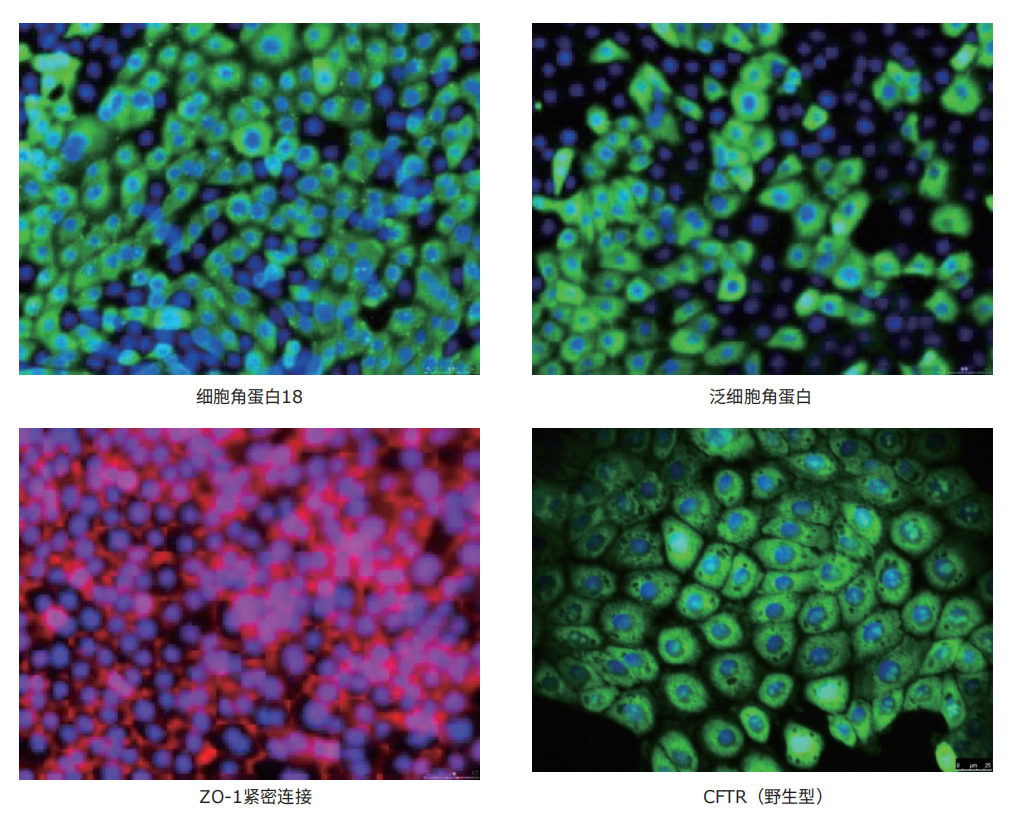
图3.16HBE14o细胞系的抗体鉴定。从左至右:16HBE14o-细胞表达肺上皮关键标志物细胞角蛋白18,泛细胞角蛋白(绿色,MAB3412),TJ蛋白ZO-1证实形成紧密连接(红色,AB2272),并表达野生型CFTR(绿色,MAB3484)。
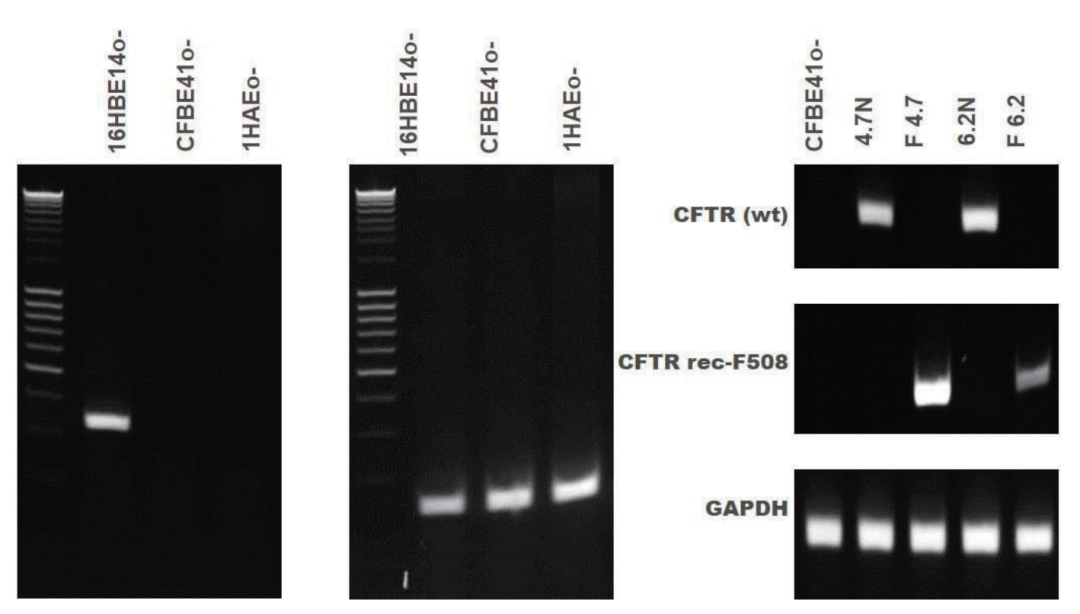
图4.支气管上皮细胞系的CFTR基因表达。16HBE14o-细胞表达野生型 CFTR,而CFBE41o-和1HAEo-缺乏CFTR基因表达。CFBE41o-细胞系来自不表达野生型CFTR的囊性纤维化患者。已产生了四个克隆,并纠正了突变(4.7N、delta F4.7、6.2N和delta F6.2)。
呼吸道病毒疾病机制(H1N1、SARS-COV、COVID-19)
支气管上皮细胞沿着进入肺部的主要通道构成功能屏障,构成抵御呼吸道病原体(包括流感 H1N1、SARS-CoV、MERS-CoV、RSV和COVID-19)的关键防线。16HBE14o 细胞系已用于研究和表征多种病毒感染机制,以支持疫苗和药物开发的研究。例如,16HBE14o-细胞表达血管紧张素转换酶2(ACE2)和丝氨酸蛋白酶 TMPRSS2,它们在SARS-CoV感染和病毒复制中起重要作用。
16HBE14o-病毒参考文献
1. Kam YW, Okumura Y, Kido H, Ng LF, Bruzzone R, Altmeyer R. 2009. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One. 4(11):e7870.2
2. Wu XL, Ju DH, Chen J, Yu B, Liu KL, He JX, et al. 2003. Immunologic mechanism of Patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLH signal pathway in vitro. Curr Microbiol. 67(4):431-6.
3. Qin L, Peng D, Hu C, Xiang Y, Zhou Y, et al. 2014. Differentiation of Th subsets inhibited by nonstructural proteins of respiratory syncytial virus is mediated by ubiquitination. PLoS One. 9(7):e101469.
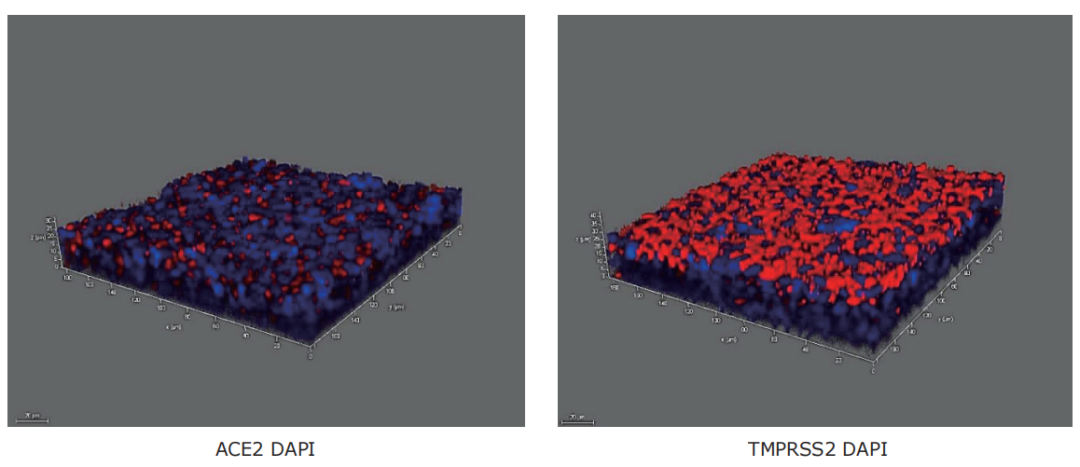
图5.16HBE细胞表达ACE-2和TMPRSS2。从左至右:用气液界面(ALI)技术培养的16HBE14o-细胞8天显示血管紧张素转换酶2(ACE2)和丝氨酸蛋白酶TMPRSS2的表达,它们在新冠病毒(SARS-CoV,COVID-19)感染和病毒复制中发挥重要作用。
三、用于哮喘和慢性阻塞性肺病研究的细胞模型
支气管哮喘和慢性阻塞性肺疾病(COPD)是阻塞性肺疾病,其特征是潜在的气道炎症,可导致呼吸困难、黏液过多和肺损伤。
16HBE14o-细胞系已用于细胞模型,以研究炎症细胞募集、激活及研究哮喘和COPD肺病的基本机制。
16HBE14o-哮喘/COPD参考文献
1. Hackett TL, de Bruin HG, Shaheen F, van den Berge M, van Oosterhout AJ, et al. 2013. Caveolin-1 controls airway epithelial barrier function.Implications for asthma. Am J Respir Cell Mol Biol. 49(4):662-71.
2. Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, et al. 2013. Barrier disrupting effects of Alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One. 8(8):e71278.
3. Pace E, Ferraro M, Minervini MI, Vitulo P, Pipitone L, et al. 2012. Beta defensin-2 is reduced in central but not in distal airways of smoker COPD patients. PLoS One. 7(3):e33601.
四、接触空气污染
汽车尾气排放和燃烧液化石油气中的臭氧(O3)、苯和二氧化氮 (NO2) 等有毒空气污染物在过敏性气道疾病和肺癌的发展中起着重要作用。16HBE14o-细胞系已作为细胞模型,以研究空气污染对人体支气管黏膜的破坏性影响。
16HBE14o-空气污染参考文献
1. Jiang CL, He SW, Zhang YD, Duan HX, Huang T, et al. 2017. Air pollution and DNA methylation alterations in lung cancer: A systematic and comparative study. Oncotarget. 8(1):1369-1391
2. Zhou Z, Liu Y, Duan F, Qin M, Wu F, et al. 2015. Transcriptomic Analyses of the Biological Effects of Airborne PM2.5 Exposure on Human Bronchial Epithelial Cells. PLoS One. 10(9):e0138267.
3. Marano F, Boland S, Bonvallot V, Baulig A, Baeza-Squiban A. 2002. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol Toxicol. 18(5):315-20.
五、吸烟和电子烟对肺功能的影响
吸烟传统香烟和电子烟(蒸汽烟草)的损害已被证明会增加上皮炎症和损伤的实例,并破坏气道上皮的宿主防御功能。此外,吸烟可损害气道上皮屏障功能,有助于肺癌的发生。
16HBE14o细胞系已被用于细胞模型研究吸烟和电子烟对人气道上皮细胞的损伤作用。
16HBE14o-吸烟参考文献
1. Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. 2012. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J. 39(2):419-28.
2. Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, et al. 2007. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. (6):748-55.
3. Gerloff J, Sundar IK, Freter R, Sekera ER2, Friedman AE. 2017. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol. 3(1):28-40.
其他细胞品类
别划走,精彩继续

长按识别
添加一对一专属技术支持

扫码进群
咨询详细产品信息

点在看,传递你的品味