【Ucallm】LongTrans In Vitro DNA Transfection Reagent 使用说明(Cat#TF07)
LongTrans In Vitro DNA Transfection Reagent
A General Protocol for Transfecting Mammalian Cell
(Cat#TF07 500 µl/1000 µl)
This product is for laboratory research ONLY and not for diagnostic use
▍▏Introduction:
Based on our innovative polymer synthesis technology, LongTrans™ DNA In Vitro Tranfection Reagent is formulated to be a powerful transfection Reagent that ensures effective and reproducible transfection with less cytotoxicity. LongTrans™ was shown to deliver genes to various established cell lines as well as primary cells.
▍▏Important Guidelines for Transfection:
LongTrans™ reagent was formulated for DNA transfection ONLY! The following standard protocol is for transfecting mammalian cells. To request protocol for lentivirus, rAAV or adenovirus production,please email us at info@ucallm.com
For better efficiency, choosing a correct protocol is essential. We strongly encourage to use “General Protocol” first. If the “General Protocol” fails to give satisfactory result (e.g., less than 10%), try the “Advanced Protocol” in the back page
For high efficiency and lower toxicity, transfect cells at high density. 70~80% confluency is highly recommended
To lower cytotoxicity, transfect cells in presence of serum (10%) and antibiotics
▍▏Part Ⅰ. A General Procedures for Transfecting Adherent Cells
Step Ⅰ. Cell Seeding:
Cells should be plated 18 to 24 hours prior to transfection so that the monolayer cell density reaches to the optimal 70~80% confluency at the time of transfection. Complete culture medium with serum and antibiotics is freshly added to each well 30~60 minutes before transfection.
Step Ⅱ. Preparation of LongTrans™-DNA Complex and Transfection Procedures:
For different cell types, the optimal ratio of LongTrans™ (µL):DNA (µg) is around 3:1. We recommend the LongTrans™ (µL):DNA (µg) ratio of 3:1 as a starting point which usually gives satisfactory transfection efficiency with invisible cytotoxicity. To ensure the optimal size of LongTrans™/DNA complex particles, we recommend using serum-free DMEM with High Glucose to dilute DNA and LongTrans™ Reagent.
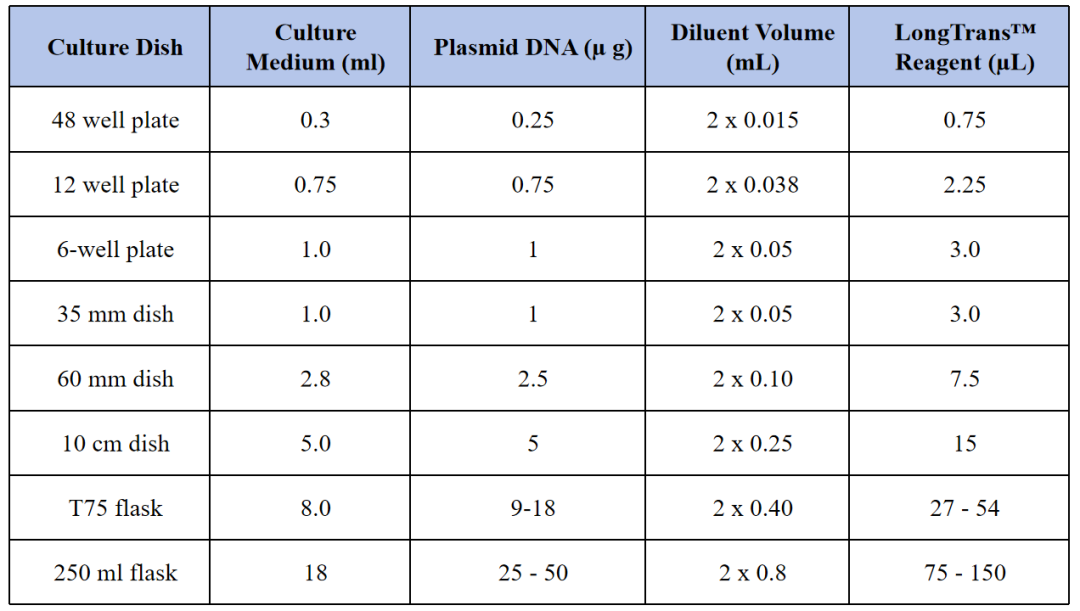
The following protocol is given for transfection in 24-well plates, refer to Table 1 for transfection in other culture formats. The optimal transfection conditions for a majority of adherent cell lines, as well as a general starting point for optimization are given in the standard protocol described below.
For each well, add 0.5 ml of complete medium with serum and antibiotics freshly 30~60 minutes before transfection.
For each well, dilute 0.5 µg of DNA into 25 µl of serum-free DMEM with High Glucose. Gently pipette up and down or vortex briefly to mix.
For each well, dilute 1.5 µl of LongTrans™ reagent into 25 µl of serum-free DMEM with High Glucose. Gently pipette up and down 3~4 times to mix.
Add the diluted LongTrans™ reagent immediately to the diluted DNA solution all at once. (Important: do not mix the solutions in the reverse order !)
Immediately pipette up and down 3~4 times or vortex briefly to mix.
Incubate for 10~15 minutes at room temperature to allow LongTrans™/DNA complexes to form.
Add the 50 µl LongTrans™/ DNA mixture drop-wise onto the medium in each well and homogenize the mixture by gently swirling the plate.
Remove LongTrans™/DNA complex-containing medium and replace with fresh complete serum/antibiotics containing medium 12~18 hours post transfection. For sensitive cells, to lower cytotoxicity, remove LongTrans™/DNA complex and replace with complete medium 5 hours after transfection.
Check transfection efficiency 24 to 48 hours post transfection.
Table 1. Recommended Amounts for Different Culture Vessel Formats
Storage: LongTrans™ Reagent is stable for up to 12 months at +4 ℃ after receipt.
LongTrans In Vitro DNA Transfection Reagent
An Advanced Protocol for Transfecting Hard-to-Transfect Cells (500 µl/1000 µl)
This product is for laboratory research ONLY and not for diagnostic use
Part Ⅱ. Advanced Protocol for Transfecting Hard-To-TransfectMammalian Cells
Important: The advanced protocol for hard-to-transfect cells is provided only if general protocol gives less than 10% efficiency. For some primary cells which cannot be trypsinized (like primary neurons), go directly to Step II, skip trypsinization and incubate freshly prepared primary cell pellet with transfection complex.
Step Ⅰ. Culturing of Cells Before Transfection:
Cells should be plated at least 24 hours prior to transfection so that the monolayer cell density reaches to the optimal 95~100% confluency at the day of transfection
Table 2. A Guideline for Optimal Cell Number Per Well in Different Culture Formats
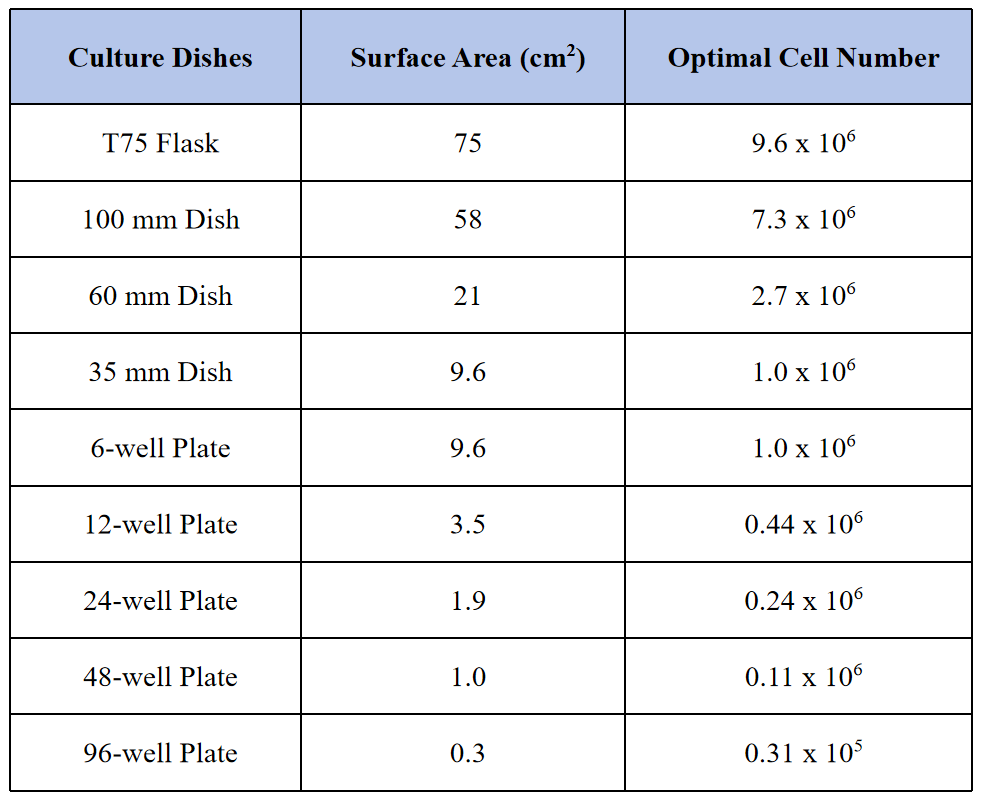
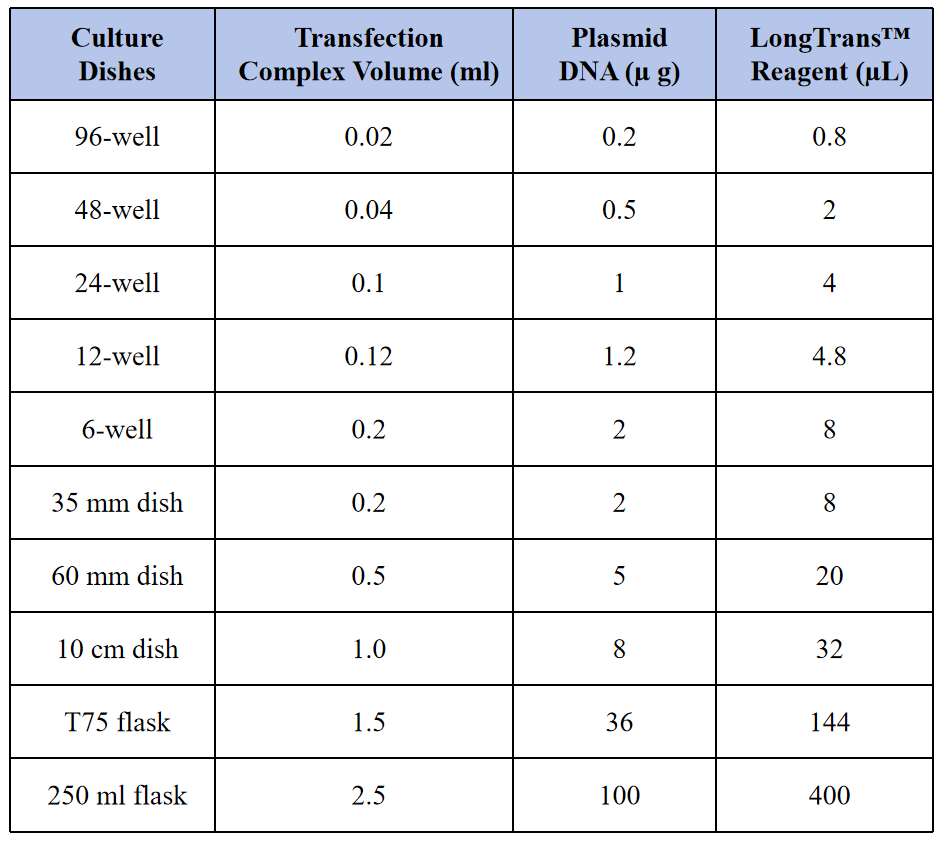
Table 3. Recommended Amounts for Different Culture Vessel Formats
Step Ⅱ. Preparation of Cells in Suspension:
The following protocol is given for transfecting hard-to-transfect cells in 6-well plates, refer to Table 2 for optimal cell number per well per Culture vessels’ surface area. The optimal transfection conditions are given in the standard protocol described below.
Detach the cells with trypsin/EDTA and stop the trypsinization with complete culture medium.
Take an aliquot of trypsinized cell suspension and count the cells to determine the cell density.
Centrifuge the required ~1.0x10 cells per well for 6-well plate at 150xg at room temperature for 10 min.
Use fine tip pipette to remove supernatant completely so that no residual medium covers the cell pellet.
Step Ⅲ. Preparation and application of Transfection Complex:
For most of mammalianells, the optimal ratio of LongTrans™ (µL):DNA (µg) is 4:1. To ensure the optimal size of complex particles, we recommend using serum-free DMEM with High Glucose to dilute DNA and LongTrans™ Reagent.
The following protocol is given for transfection in 6-well plates, refer to Table 3 for transfection in other culture formats.
For each well of 6-well plate, dilute 2 µg of DNA into 100 µl of serum-free DMEM with High Glucose. Vortex gently and spin down briefly to bring drops to bottom of the tube.
For each well of 6-well plate, dilute 8 µl of LongTrans™ reagent into 100 µl of serum-free DMEM with High Glucose. Vortex gently and spin down briefly
Add the diluted LongTrans™ Reagent immediately to the diluted DNA solution all at once. (Important: do not mix the solutions in the reverse order !)
Immediately pipette up and down 3~4 times or vortex briefly to mix followed by incubation for ~15 minutes at room temperature to allow LongTrans™/DNA transfection complexes to form.
Gently resuspend the cell pellet prepared from Step II immediately in the 200 µl transfection complex and incubate at 37 °C for 20 minutes.
At the end of incubation, add 2.0 ml of pre-warmed fresh complete cell growth medium to cells and plate onto one well of a 6-well plate. Incubate at 37 ℃ with 5% CO2
Remove transfection complex containing medium gently and refill with complete culture medium 8~12 hours after plating.
Check transfection efficiency 24 to 48 hours post transfection.
点在看,传递你的品味