rPeptide是美国一家生物化学研发公司,主要产品涉及领域为老年性痴呆症与帕金森综合症研究使用的重组蛋白,重组多肽,抗体以及试剂等等,另外还提供一系列外包服务,从分子生物学,蛋白表达与蛋白纯化到13C与15N 统一标记蛋白与多肽。同时, rPeptide 技术平台可解决可溶性多肽/蛋白(例如:β-淀粉样蛋白、廋蛋白、前胰岛素)在大肠杆菌中表达等历史性难题。
以下是该公司部分代表产品:
Antibodies(抗体)

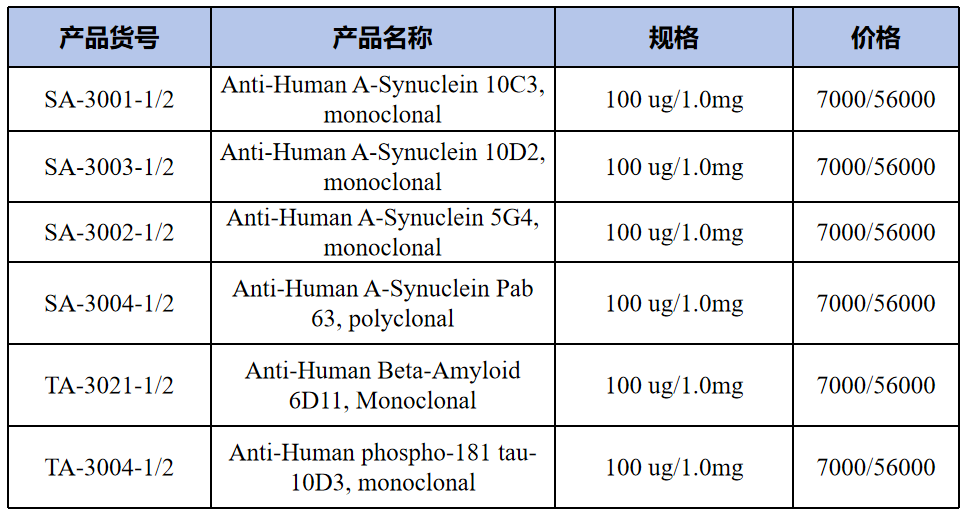
Peptides(多肽类)

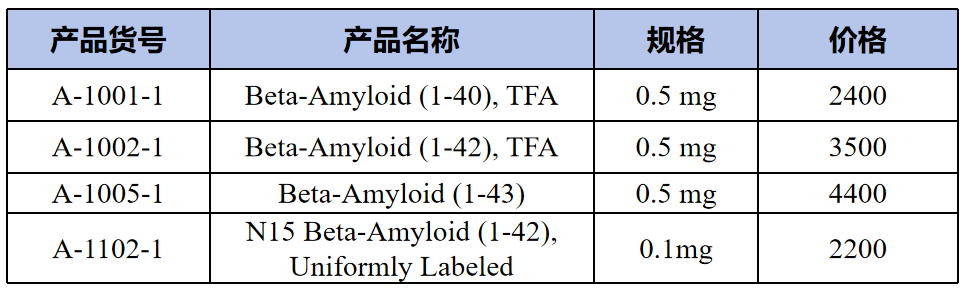
Proteins(蛋白质)

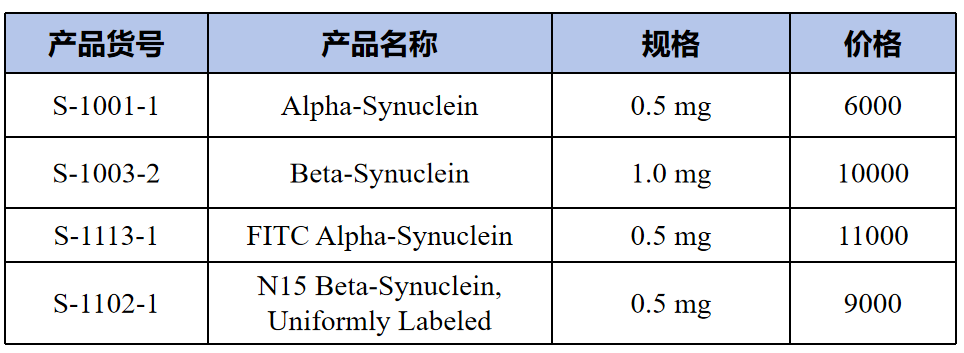
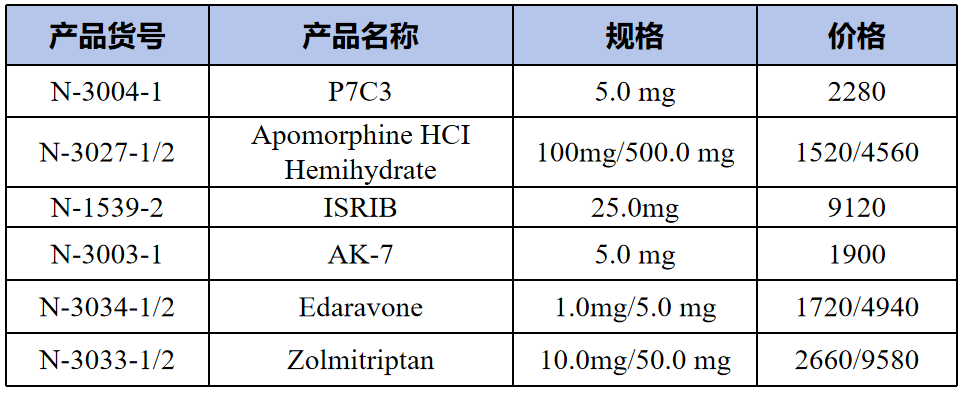
Neurodegenerative related compounds(神经退行性相关化合物)
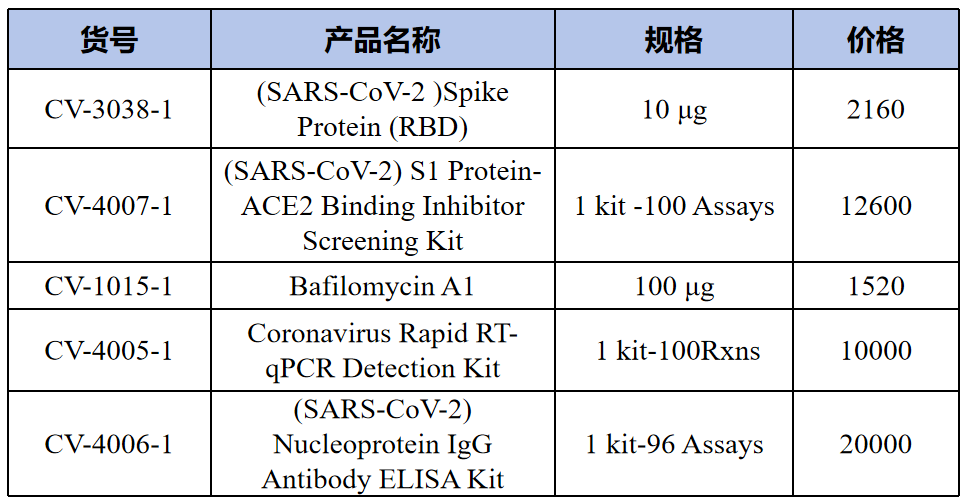
Coronavirus research tools(冠状病毒研究工具)
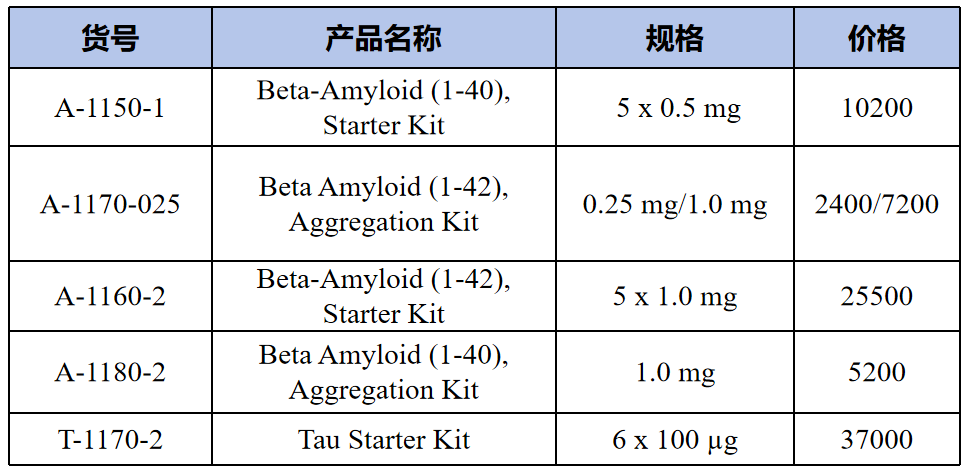
Kits(试剂盒)

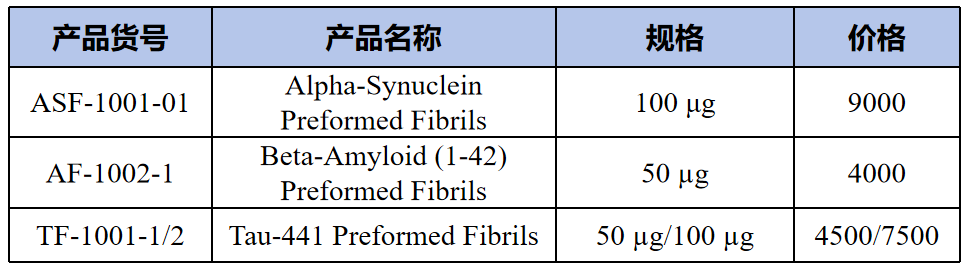
Preformed fibrils(预成型的纤维)
rPeptide 产品列表
Proteins
| 货号 | 名称 | 规格 | 产品描述 |
| S-1001-1 | Alpha-Synuclein | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. |
| S-1001-2 | Alpha-Synuclein | 1.0 mg | |
| S-1004-1 | N15 Alpha-Synuclein, Uniformly Labeled | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. N15 uniformly labeled alpha-synuclein (α-synuclein) has all nitrogen’s N15 labeled. |
| S-1004-2 | N15 Alpha-Synuclein, Uniformly Labeled | 1.0 mg | |
| S-1002-1 | Alpha-Synuclein, A53T Mutant | 0.5 mg | lpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A point mutant (A53T) of alpha-synuclein gene that has been linked to autosomal dominant early onset Parkinson’s Disease (PD). |
| S-1002-2 | Alpha-Synuclein, A53T Mutant | 1.0 mg | |
| S-1005-1 | Alpha-Synuclein, A30P Mutant | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A point mutant (A30P) of α-Synuclein gene that has been linked to autosomal dominant early onset Parkinson’s Disease (PD). |
| S-1005-2 | Alpha-Synuclein, A30P Mutant | 1.0 mg | |
| S-1006-1 | Alpha-Synuclein, A30P, A53T Mutant | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A double mutant (A30P/A53T) of α-Synuclein gene that has been linked to autosomal dominant early onset Parkinson’s Disease (PD). |
| S-1006-2 | Alpha-Synuclein, A30P, A53T Mutant | 1.0 mg | |
| S-1008-1 | Alpha-Synuclein, E46K Mutant | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A point mutant (E46K) of α-Synuclein gene that has been linked to autosomal dominant early onset Parkinson’s Disease (PD). |
| S-1008-2 | Alpha-Synuclein, E46K Mutant | 1.0 mg | |
| S-1015-1 | Alpha-Synuclein, Delta-NAC | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A deletion mutant of α-synuclein that lacks the NAC region (amino acid 61-95). 6 amino acids were added as a linker. |
| S-1016-1 | Alpha-Synuclein, 112 (NACP 112) | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. An alternatively spliced (103-129) form of α-synuclein. |
| S-1017-1 | Alpha-Synuclein, S9C Mutant | 0.5 mg | This human alpha-synuclein (α-Synuclein) contain a mutation at S9C that can be used for thiol coupling or thiol modification. The S9C mutant is compatible with haloalkyl derivatives, maleimides, and sulfonates. Possible applications include fluorescence labeling 1, spin labeling for EPR 2, or PRE experiments |
| S-1017-2 | Alpha-Synuclein, S9C Mutant | 1.0 mg | |
| T-1001-1 | Tau-441 | 50 µg | Tau is a family of major neuronal microtubule associated proteins that are found in the neurofibrillary tangles (NFT) in Alzheimer’s disease. Tau promotes the assembly and maintains the structure of microtubules in neuronal cells. The Tau proteins are derived from alternative mRNA splice variants that originate from a single gene and result in mature proteins that vary in size from 352 to 441 amino acids (36.8 to 45.9 kDa). There are six Tau isoforms, that differ from one another in having three or four microtubule binding repeats (R) of 31-32 amino acids each, and two, one or none amino terminal inserts (N) of 29 amino acids each. While the fetal brain contains a single isoform of tau (Tau-352) the adult brain has several isoforms all derived from a single gene by alternative mRNA splicing. |
| T-1001-2 | Tau-441 | 100 µg | |
| A-2002-1 | Alpha 1 Antichymotrypsin, Human Plasma | 100 µg | AD patients seem to express increased alpha 1-Antichymotrypsin (AACT) index, suggesting an intrathecal production of AACT. Indeed, AACT is a major component of the amyloid plaques found in the brains of AD patients. In Parkinson’s disease (PD), AACT gene has been suggested as a susceptibility factor that might be related to the onset of PD. |
| A-2003-1 | Alpha 1 Antitrypsin, Human Plasma | 1.0 mg | Like alpha 1-antichymotrypsin alpha 1-antichymotrypsin might be functionally involved in the pathogenesis of the lesions of Alzheimer’s disease. Both serine protease inhibitors are found in neurofibrillary tangles and senile plaques. The major cell producing alpha 1-antitrypsin localization in seems to be the astrocytes, which are involved in both lesions. |
| A-2004-1 | Alpha 2 Macroglobulin, Human Plasma | 1.0 mg | Alpha 2 Macroglobulin (A2M) seems to play a role in the development of sporadic AD. A2M-D allele was observed as a weak AD protective factor, and the possible interaction of APOE-epsilon 4 and A2M-G alleles may have cause an “increase AD risk in Mainland Han Chinese”. |
| S-1010-1 | Alpha-Syncuclein, Mouse | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. Mouse alpha-synuclein has a 95% homologous sequence to human alpha-synuclein. Alpha-synuclein fibrils are prevalent in Lewy bodies, a physiological symptom of Parkinson’s Disease. |
| S-1010-2 | Alpha-Syncuclein, Mouse | 1.0 mg | |
| S-1011-1 | Alpha-Synuclein, 1-60 | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A deletion mutant of α-synuclein (amino acids 1-60), which contains the N-terminal amphipathic domain. |
| S-1012-1 | Alpha-Synuclein, 1-95 | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A deletion mutant of α-synuclein (amino acids 1-95), which contains the N-terminal amphipathic domain and the NAC region. |
| S-1013-1 | Alpha-Synuclein, 61-140 | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A deletion mutant of α-synuclein (amino acids 61-140). Additional amino acid (Met) is attached at the N-terminus. |
| S-1014-1 | Alpha-Synuclein, 96-140 | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. A deletion mutant of α-synuclein (amino acids 96-140). Additional amino acid (Met) is attached at the N-terminus. |
| S-1153-1 | Alpha-Synuclein, Desalted | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. |
| S-1153-2 | Alpha-Synuclein, Desalted | 1.0 mg | |
| S-1021-1 | Alpha-Synuclein, Monkey | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. Monkey alpha-synuclein has a 98% homologous sequence to human alpha-synuclein. |
| S-1021-2 | Alpha-Synuclein, Monkey | 1.0 mg | |
| S-1022-1 | Alpha-Synuclein, Monkey, Desalted | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. Monkey alpha-synuclein has a 98% homologous sequence to human alpha-synuclein. This product is supplied in a buffer without salt to better fill the needs of customers whose experiments may have interference with NaCl. |
| S-1022-2 | Alpha-Synuclein, Monkey, Desalted | 1.0 mg | |
| S-1020-1 | Alpha-Synuclein, Mouse, Desalted | 0.5 mg | Alpha-Synuclein (α-Synuclein) is a 14 kD (140 amino acids) acidic presynaptic protein and is a major component of Parkinson’s Disease (PD) aggregates. α-Synuclein is implicated in the pathogenesis of PD and related neurodegenerative disorders. α-Synuclein also accumulates in the brains of sporadic PD patients as a major component of Lewy bodies, which are intraneuronal cytoplasmic inclusions characteristic of PD. α-Synuclein appears to associate with other proteins that aggregate and is found in beta-amyloid plaques and neuritic tangles in Alzheimer’s disease 3. rPeptide offers a wide array of synuclein products, including fragments, mutants, preformed fibrils, and labeled versions in addition to the wild-type protein. Mouse alpha-synuclein has a 95% homologous sequence to human alpha-synuclein. Alpha-synuclein fibrils are prevalent in Lewy bodies, a physiological symptom of Parkinson’s Disease. This product is supplied in a buffer without salt to better fill the needs of customers whose experiments may have interference with NaCl. |
| S-1020-2 | Alpha-Synuclein, Mouse, Desalted | 1.0 mg | |
| A-2011-1 | Apo-D, human recombinant | 10 µg | Apolipoprotein-D (Apo-D) is mainly associated with high density lipoproteins in human plasma. It is expressed in numerous tissues having high levels of expression in spleen, testes and brain. Apo-D is a multiligand, multi-functional transporter and transports a ligand from 1 cell to another within an organ, scavenge a ligand within an organ for transport to the blood or could transport a ligand from the circulation to specific cells within a tissue. The recombinant human Apo-D expressed from E.Coli is a single, non-glycosylated, Polypeptide chain containing 174 amino acids and having a molecular mass of 19.8 kDa |
| A-2012-1 | APO-J / Clusterin | 10 µg | Apoliprotein J (APO-J), also named Clusterin, is a 75-80 kD disulfidelinked heterodimeric protein. The precursor polypeptide chain is cleaved proteolytically to remove the 22-mer secretory signal peptide and subsequently between residues 227/228 to generate the α and β chains. These are assembled in anti-parallel to give a heterodimeric molecule in which the cysteine-rich centers are linked by five disulfide bridges and are flanked by two predicted coiled-coil a-helices and three predicted amphipathic α -helices. Clusterin is up- or down regulated on the mRNA or protein level in many pathological and clinically relevant situations including cancer, organ regeneration, infection, Alzheimer disease, retinitis pigmentosa, myocardial infarction, renal tubular damage, autoimmunity and others |
| A-2013-1 | Apo-SAA, human recombinant | 10 µg | Human Apo-SAA is a 104 amino acid polypeptide that circulates primarily in association with high-density lipoproteins (HDL). The level of ApoSAA, normally 1-5 µg/ml in plasma, increases 500-1000-fold within 24 hours of an inflammatory stimulus and, under these conditions, is the most abundant HDL apo-lipoprotein. The human SAA gene codes for a 122 amino acid polypeptide, which contains an 18 amino acid N-terminal signal sequence. |
| A-2013-2 | Apo-SAA, human recombinant | 50 µg | |
| A-2013-3 | Apo-SAA, human recombinant | 500 µg | |
| A-2014-1 | ApoA-1, human recombinant | 20 µg | ApoA-I is a 29.0 kDa protein produced in the liver and intestine, and secreted as the predominant constituent of nascent high-density lipoprotein (HDL) particle. ApoA-I, which is found exclusively in HDL, has a unique ability to capture and solubilize free cholesterol. This apoA-I ability enables HDL to remove excess peripheral cholesterol and return it to the liver for recycling and excretion. This process, called reverse cholesterol transport, is thought to inhibit atherogenesis. For this reason, HDL is also known as the “good cholesterol.” The therapeutic potential of apoA-I has been recently assessed in patients with acute coronary syndromes, using a recombinant form of a naturally occurring variant of apoA-I (called apoA-I Milano). The availability of recombinant normal apoA-I should facilitate further investigation into the potential usefulness of apoA-I in preventing atherosclerotic vascular diseases. Recombinant human ApoA-I is a 28.2 kDa protein of 244 amino acid residues. |
| A-2014-2 | ApoA-1, human recombinant | 100 µg | |
| A-2014-3 | ApoA-1, human recombinant | 1.0 mg | |
| A-2015-1 | ApoE2, human recombinant | 50 µg | ApoE belongs to a group of proteins that bind reversibly with lipoprotein and play an important role in lipid metabolism. In addition to facilitating solublization of lipids, these proteins help to maintain the structural integrity of lipoproteins, serve as ligands for lipoprotein receptors, and regulate the activity of enzymes involved in lipid metabolism. Significant quantities of ApoE are produced in liver and brain and to some extent in almost every organ. ApoE is an important constituent of all plasma lipoproteins. It’s interaction with specific ApoE receptor enables uptake of chylomicron remnants by liver cells, which is an essential step during normal lipid metabolism. It also binds with the LDL receptor (apo B/E). Defects in ApoE are a cause of hyperlipoproteinemia type III. ApoE exists in three major isoforms; E2, E3, and E4, which differ from one another by a single amino-acid substitution. Compared with E3 and E4, E2 exhibits the lowest receptor binding affinity. E2 allele carriers had significantly lower levels of total cholesterol, low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol, as well as increased ApoE levels. Recombinant human ApoE2 is a 34.3 kDa protein containing 300 amino acid residues. |
| A-2015-2 | ApoE2, human recombinant | 100 µg | |
| A-2015-3 | ApoE2, human recombinant | 500 µg | |
| A-2015-4 | ApoE2, human recombinant | 1.0 mg | |
| A-2015-5 | ApoE2, human recombinant | 5.0 mg | |
| A-2016-1 | ApoE3, human recombinant | 50 µg | ApoE belongs to a group of proteins that bind reversibly with lipoprotein and play an important role in lipid metabolism. In addition to facilitating solublization of lipids, these proteins help to maintain the structural integrity of lipoproteins, serve as ligands for lipoprotein receptors, and regulate the activity of enzymes involved in lipid metabolism. Significant quantities of ApoE are produced in liver and brain and to some extent in almost every organ. ApoE is an important constituent of all plasma lipoproteins. It’s interaction with specific ApoE receptor enables uptake of chylomicron remnants by liver cells, which is an essential step during normal lipid metabolism. It also binds with the LDL receptor (apo B/E). Defects in ApoE are a cause of hyperlipoproteinemia type III. ApoE exists in three major isoforms; E2, E3, and E4, which differ from one another by a single amino-acid substitution. Compared with E3 and E4, E2 exhibits the lowest receptor binding affinity. E2 allele carriers had significantly lower levels of total cholesterol, low-density lipoprotein cholesterol, and non-high-density lipoprotein cholesterol, as well as increased ApoE levels. Recombinant human ApoE2 is a 34.3 kDa protein containing 300 amino acid residues. |
| A-2016-2 | ApoE3, human recombinant | 100 µg | |
| A-2016-3 | ApoE3, human recombinant | 500 µg | |
| A-2016-4 | ApoE3, human recombinant | 1.0 mg | |
| A-2016-5 | ApoE3, human recombinant | 5.0 mg | |
| A-2017-1 | ApoE4, human recombinant | 50 µg | ApoE belongs to a group of proteins that bind reversibly with lipoprotein and play an important role in lipid metabolism. In addition to facilitating solublization of lipids, these proteins help to maintain the structural integrity of lipoproteins, serve as ligands for lipoprotein receptors, and regulate the activity of enzymes involved in lipid metabolism. Significant quantities of ApoE are produced in liver and brain and to some extent in almost every organ. ApoE is an important constituent of all plasma lipoproteins. It’s interaction with specific ApoE receptor enables uptake of chylomicron remnants by liver cells, which is an essential step during normal lipid metabolism. It also binds with the LDL receptor (apo B/E). Defects in ApoE are a cause of hyperlipoproteinemia type III. ApoE exists in three major isoforms; E2, E3, and E4, which differ from one another by a single amino-acid substitution. Individuals heterozygous for the ApoE4 allele are at higher risk of late-onset Alzheimer’s disease. Recombinant human ApoE4 is a 34.4 kDa protein containing 300 amino acid residues. |
| A-2017-2 | ApoE4, human recombinant | 100 µg | |
| A-2017-3 | ApoE4, human recombinant | 500 µg | |
| A-2017-4 | ApoE4, human recombinant | 1.0 mg | |
| A-2017-5 | ApoE4, human recombinant | 5.0 mg | |
| A-2001-1 | Apolipoprotein E, Human Plasma, VLDL | 50 µg | Apolipoprotein E, (Apo E) is present in normal plasma at concentrations of 50 ug/ml. Apo E serves as a ligand for LDL receptors, where it participates in the transport and redistribution of cholesterol and other lipids. Other functions include immunoregulation, cell growth, and differentiation. Thought to be involved in tissue repair. |
联系我们
相关推荐
评论列表共有 0 条评论
暂无评论
发表评论
取消回复
药科美专注生命科学产品进出口与技术服务