【Zenogen Pharma】HSC-BANKER® GMP grade
Description
(For cryopreserving hematopoietic stem cell)
HSC-BANKER® GMP grade is an optimized cryopreservation medium for hematopoietic stem cells.
It is a ready-to-use product and has been reported that the cryopreservation outcomes of HSC-BANKER® is at least equivalent to those of the conventional protocol using DMSO and DEXTRAN.
It is completely free of serum and animal derived component, and contains only USP, EP, JP graded ingredients or JP excipients. HSC-BANKER® GMP grade is manufactured in a facility compliant with JP, EU, US and PIC/S GMP guidelines and the production and the quality are controlled in compliance with JP GMP guidelines.
※This product has been registered in MF within PMDA in 2019.
Package:15ml/bottle
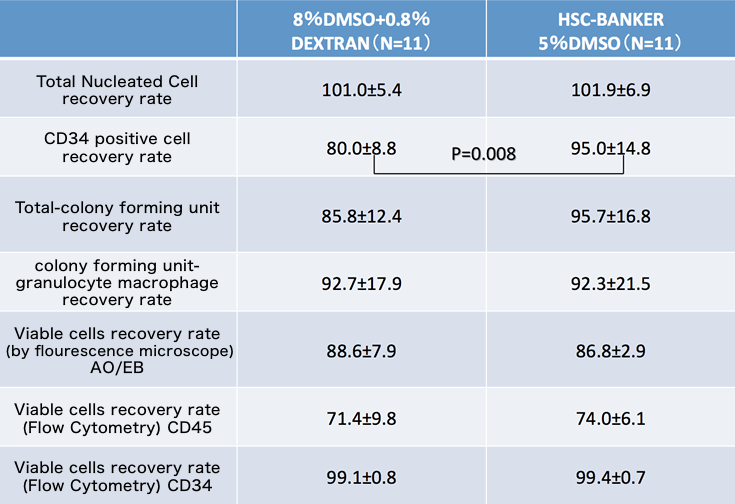
Comparative study
This data is provided from Japan Red Cross Society.
Freezing Protocol
1.Remove red blood cells from the cord blood collection.
2.Separate the cord blood into plasma and buffy coat fractions centrifugation at 400 x g for 10 minutes.
3.Reduce the volume of the cord blood to 13 mL removing the plasma.
4.Add gently an equal volume (13 mL) of HSC-BANKER® GMP grade to the cord blood, and place the freezing bag in a controlled rate freezer to gradually freeze the cord blood to -80℃.
5.After the cord blood has reached the temperature of below -80°C, transfer it to a liquid nitrogen tank for long term storage.
Storage and Stability
Store at 2 to 8 degrees Celsius. In unopened condition and under storage temperatures (2-8℃), the products will be stable for 3 years, after the date of manufacture at this storage temperature.
其他细胞品类
别划走,精彩继续

长按识别
添加一对一专属技术支持

扫码进群
咨询详细产品信息

点在看,传递你的品味