【Megazyme】Enzymes and Beermaking
Streamlining Enzyme Analysis in Brewing
Technical advancements have been applied to all stages of beer production, from cereal harvesting to filtration to bottling and packaging. All advancements have moved towards the same solution: automation.
While other aspects of beermaking have modernised, analytical aspects of brewing science have arguably lagged behind: maltsters and brewers continue to use analytical methodologies developed in the 19thand early 20thcenturies.
▍▏The traditional approach to malt and beer analysis
Malts usually come with a detailed passport including a long list of properties and parameters. Despite the reliance of cereal breeders, maltsters, and brewers on these descriptions, many of the measurements included are based on traditional methods that lack specificity and sensitivity. Others require specialised equipment or are highly labour-intensive, making them ill-suited to automation.
Brewers are typically comfortable with the methods they trained to use at the start of their careers, and are understandably reluctant to abandon them.
However, a number of new analytical methods are worth considering as substitutes for (or additions to) the traditional practices, offering the user new information on properties that are important to brewing. Importantly, these substitutes also offer the potential for brewers to automate the analysis of malts and beers.
▍▏Enzymes and beer
There are two main biochemical processes central to brewing which require enzymes:
1. conversion of starch (naturally present in barley) into fermentable sugars, and
2. fermentation of sugars by yeasts to produce ethanol and carbon dioxide.
Starch conversion involves two classes of enzyme - cell wall hydrolases and starch hydrolases - which are released by the barley grain itself during malting.

Like most cereals, barley contains starch as its primary storage polysaccharide. Starch must be released from inside the barley endosperm, then broken down into maltose and glucose before it becomes useful for brewing.
The natural process of germination involves the release of enzymes that allow the growing seed to break down its endosperm cell walls and access its starch reserves.
Maltsters take advantage of this process, germinating the barley grain under controlled conditions in such a way that the essential endogenous enzymes are produced and released.
Malting is terminated by drying with hot air (‘kilning’), which kills the barley embryo and prevents further starch loss via respiration. However, unavoidably, some of the enzymes produced during malting are deactivated by high temperatures at kilning.
Brewers become involved from this stage onwards. They combine the malts with water and (optionally) adjuncts, then begin cooking the mixture in a process called mashing.
Mashing aims to complete the destruction of the cell wall and accelerate the breakdown of starch. Heat is applied according to a pre-established programme intended to maximise the activity of the remaining enzymes.
Once the cell wall is broken down, starch hydrolases are able to reach the starch molecules and break them into smaller fragments - the smallest being the fermentable sugars maltose and glucose.

Other important molecules such as amino acids - which comprise the majority of the free amino nutrients (FAN) - are also formed at this stage, mainly due to the action of protease enzymes. FAN plays an important role in feeding yeast during the fermentation stage of beer production.
Next comes the second enzyme-intensive process in beermaking: fermentation. Yeasts release enzymes to convert sugars into ethanol and carbon dioxide. However, the fermentability and filterability of the final beer is largely predecided, based on the enzymatic activity that occurred in malting and mashing.
▍▏Measuring enzyme activity
With enzymes playing such a critical role in the brewing process, maltsters and brewers must be able to measure enzyme activity in their raw materials. Only by understanding the enzymatic potential of their malts can maltsters and brewers fully exploit the enzymatic processes that influence the consistency and quality of their beer.
Brewers seeking to maximise particular attributes of their beer may add exogenous enzymes to promote relevant enzyme activity.
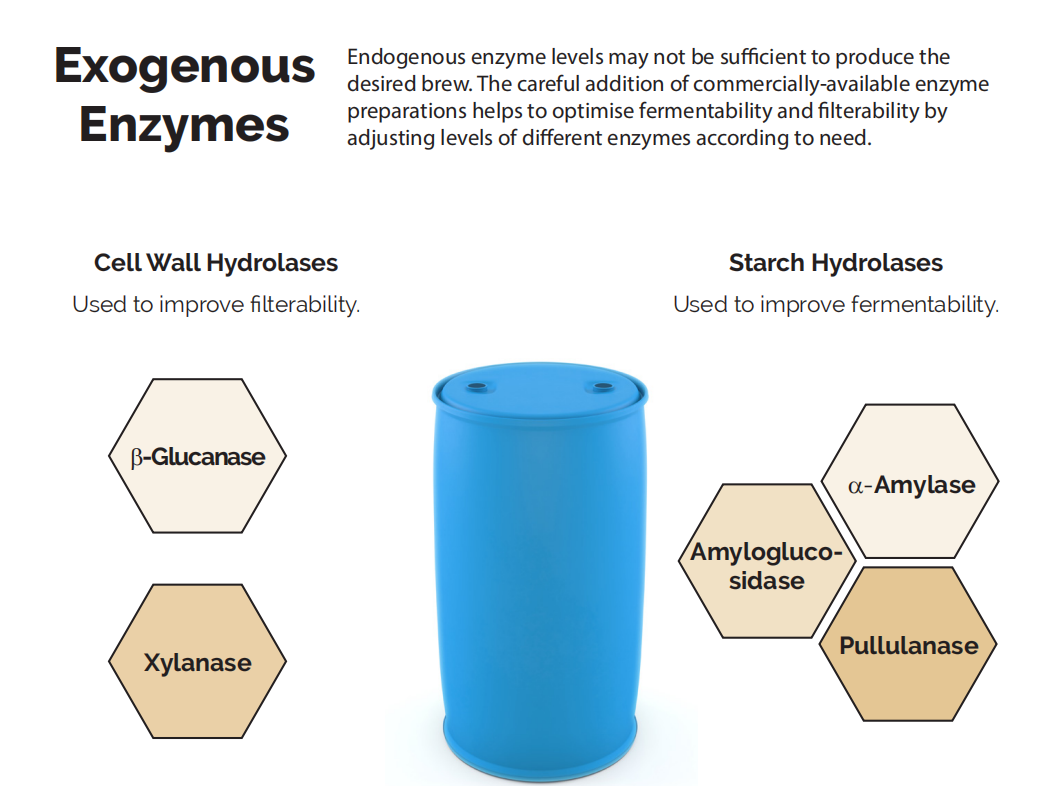
A brewer may use exogenous enzyme supplementation if there is concern that endogenous enzyme levels will not be sufficient. Barley variety, pre-harvest sprouting and methods of malting, kilning and mashing may all influence endogenous enzyme levels.
Exogenous enzyme mixtures can correct issues like stuck mashes and low extract yields. These enzymes also enable beermakers to brew efficiently with unmalted cereal adjuncts (e.g. corn, wheat and rice) to produce light beers or gluten-free beers.
However, exogenous enzymes should not be viewed as a ‘quick-fix’ solution that can be undertaken lightly: while one enzyme carries out the desired activity, the presence of others may result in undesired hydrolytic activities with the potential to affect the final product, e.g. addition of protease (such as papain) to beer to address haze formation, with the undesired result of inferior foam stability unless dosage is carefully controlled.
To add an unnecessarily large amount of exogenous enzyme may be costly, counterproductive or simply futile.
Conversely, the addition of too little exogenous enzyme may fail to address delays in the brewing process
▍▏Starch hydrolases
To be useful for brewing, starch must be broken down into smaller units, such as maltose and glucose, which can later be used by yeasts during fermentation. The enzymes responsible for breaking down the long starch chains into fermentable sugars are called starch hydrolases.
Four main enzymes are involved in the transformation, all of which are produced or activated during malting. All are necessary in order to maximise the fermentability of the brew:
1. α-Amylase
2. β-Amylase
3. Limit-Dextrinase
4. α-Glucosidase
Each has its own specific role to play and a unique pattern of action, which will be discussed in turn. α-Glucosidase is present at such low levels that its contribution to the brewing process is not considered in detail here.
Starch itself is divided into two structural forms. Amylose is composed of relatively linear α-1,4-glucose chains, while amylopectin is a branched polysaccharide in which α-1,4- glucose chains are joined together by α-1,6 branch points.

An important property of starch is its gelatinisation temperature, that is, the minimum temperature that must be reached during mashing. On reaching the gelatinisation temperature in the presence of water, the tight crystalline structure of the starch granule is destroyed, making the polysaccharide readily accessible to starch hydrolases.
The gelatinisation temperature is a crucial concept in mashing as it determines the persistence and activity of all hydrolases, some of which are more thermostable than others. This temperature depends on a number of factors (including amylose:amylopectin ratio, the cereal used, and the use of adjuncts) but is typically > 60oC.
α-Amylase
α-Amylase is an endo-acting enzyme that randomly cuts internal α-1,4 linkages in the starch molecules. This has two main effects: firstly, the viscosity of the mash drops rapidly, and secondly, maltodextrins are produced.

As the most thermostable of the starch hydrolases, α-amylase has a temperature optimum up to 70oC which allows it to tolerate the temperatures involved in starch gelatinisation. Around 90% of α-amylase persists through the mashing phase. Therefore, the amount of α-amylase that persists after mashing is generally not the limiting factor in obtaining the maximal hydrolysis of starch.
β-Amylase
Like α-amylase, β-amylase is able to cut α-1,4 linkages in starch chains. However, β-amylase is an exo-acting enzyme, which means that instead of randomly cutting internal linkages in starch, it cuts from the end of the chain only.

β-Amylase liberates fermentable maltose molecules, which account for ~ 65% of the fermentable sugar in wort. α-Amylase and β-amylase are able to work synergistically during mashing, as β-amylase generally acts on the maltodextrin fragments liberated by the initial hydrolytic action of α-amylase.
β-Amylase is the most abundant starch hydrolase during malting, however it is significantly less thermostable than α-amylase. A recent study found that just 40% of the initial β-amylase persisted to continue liberating maltose in the mash. Brewers therefore need to quantify the true activity of β-amylase: the presence of too little β-amylase has the potential to limit the fermentability of the resulting wort. This is a particular concern when brewing with adjuncts.
With all of this in mind, it seems a significant oversight that specific β-amylase measurement is not carried out routinely. Although simple and effective analytical solutions exist, there is currently no specific method recommended by official bodies for measurement of β-amylase.
Limit-Dextrinase
Every team has a specialist individual that can carry out a delicate task that is outside others’ area of expertise. Among the starch hydrolases, limit-dextrinase has the unique capability to cut α-1,6 linkages - that is, the bonds that are contained in the ‘branched’ portions of amylopectin chains.
Branched maltodextrins cannot be hydrolysed by the other starch hydrolases. The activity of limit-dextrinase converts amylopectin chains into shorter, unbranched maltodextrins that can be broken down further by α-amylase and β-amylase into fermentable sugars.

Continued presence of branched maltodextrins negatively influences the fermentability of the resulting wort and also impacts on the mouthfeel of the finished beer. Limitdextrinase is of particular importance for brewers using certain cereal adjuncts, especially rice in Asian beers. Careful control of starch hydrolases in general - but limit-dextrinase in particular - is needed to ensure satisfactory fermentability.
Limit-dextrinase is mainly produced during malting, albeit in much smaller quantities than either α- or β-amylase. Like β-amylase, limit-dextrinase is relatively heat-sensitive: ~ 40% of limit-dextrinase remains in mash after 60 minutes.
A further starch hydrolase called pullulanase is often added to mashes as an exogenous enzyme. Pullulanase has a similar activity pattern to limit-dextrinase, helping to break branching points and promote fermentability. This enzyme is of particular interest to brewers producing ‘light’ beers with a lower calorific content.
▍▏Cell wall hydrolases
Often overlooked by maltsters and brewers, cell wall hydrolases are every bit as important as starch hydrolases. Cell wall hydrolases break down the cell walls that enclose the grain endosperm. This process allows starch hydrolases to access the starch inside.
Endosperm cell walls in barley are made up of non-starch polysaccharides. β-Glucan accounts for ~ 75%, a further ~20% is arabinoxylan (pentosan) with the remainder largely cellulose.
The most important enzymes involved in cell wall hydrolysis are all produced during malting and become most important during mashing:

1. β-glucanase
2. xylanase
3. cellulase
The relative presence or absence of cell wall hydrolases in malt has a strong influence on a brewer’s process decisions. The use of undermodified malts leads to incomplete cell wall hydrolysis. This has a direct impact on wort viscosity, lautering performance, beer filtration and the likelihood of haze. There is also a potential knock-on effect on extract yield and wort fermentability.
Where brewers are aware of low hydrolase concentrations, these effects can be mitigated during the production process. For example, a brewer could choose mashing programmes which employ lower temperatures, promoting the persistence and activity of cell wall hydrolases.
Malt β-Glucanase
β-Glucanase is an endo-acting enzyme that cuts internal β-1,4 bonds in the β-glucan chain. Demonstrating remarkable specificity, β-glucanase can only hydrolyse β-1,4 bonds adjacent to and on the reducing side of the β-1,3 linkage.
It is also able to hydrolyse β-glucans that are bound to proteins, which contribute to increased wort viscosity and can cause precipitates and haze formation if left unaddressed.
β-Glucanase is primarily active during the malting stage; some enzyme is denatured at kilning, while the rest becomes inactive at temperatures close to starch gelatinisation. For this reason, exogenous β-glucanase is a common addition to mash.

Xylanase
Arabinoxylan - also known as pentosan - is the second most abundant polysaccharide in barley endosperm cell walls. Its structure contains two main sugars: arabinose and xylose.
The latter constitutes the xylan ‘backbone’ of the polysaccharide and is linked via β-1,4 bonds, while arabinose is appended to the xylan chain via α-linkages. Wheat has a particularly high arabinoxylan content. Xylanase is the endo-acting enzyme responsible for cleaving arabinoxylan. It does so by hydrolysing the β-1,4 bonds that link the xylose residues.

Xylanase is found at very low concentrations in malts, and becomes even scarcer by the time malts reach the mash tun: like β-glucanase, xylanase exhibits poor thermostability, with a portion becoming deactivated during kilning.
Where xylanase activity is low, soluble arabinoxylans persist into the wort, causing high viscosity, poor filterability, and the formation of beer hazes. Xylanase activity is therefore an important parameter for brewers to measure, particularly those using processes that prohibit the use of exogenous enzymes. Unsurprisingly, xylanase is also a very common component of exogenous enzyme preparations for brewers.
Cellulase
Cellulase is an endo-acting enzyme which hydrolyses the β-1,4 bonds present in cellulose and β-glucan. Alongside β-glucanase and xylanase, cellulase is also involved in starch mobilisation. Therefore, its level in malt has a knock-on effect on mash viscosity and starch accessibility.

Assay Kits for the Measurement of Enzyme Activity
▍▏Starch hydrolases
Measure starch hydrolases to ensure starch is completely broken down during mashing. This maximises fermentability in the subsequent wort.
α-Amylase

Product Code |
Product Name |
Assays per Kit |
K - AMYLSD |
α - Amylase SD (High Sensitivity) Assay Kit |
160 |
K - CERA |
Ceralpha Assay Kit |
100 |
THREE DIFFERENT APPROACHES TO α-AMYLASE ANALYSIS

β-Amylase
Product Code |
Product Name |
Assays per Kit |
K - BETA3 |
Betamyl - 3 Assay Kit |
100 |
α-Amylase and β-Amylase
Product Code |
Product Name |
Assays per Kit |
K - MALTA |
Malt Amylase Assay Kit (Contains 50 Ceralpha assays and 50 Betamyl - 3 assays.) |
100 |
Pullulanase and Limit-Dextrinase
Product Code |
Product Name |
Assays per Kit |
K - PullG6 |
Pullulanase/Limit - Dextrinase Assay Kit |
100 |
▍▏Cell wall hydrolases
Measure cell wall hydrolases to ensure maximal starch mobilisation during malting and mashing. This aids filterability by reducing the viscosity of the resulting wort, and has repercussions for fermentability by making starch available to starch hydrolases.
β - Glucanase | ||
Product Code |
Product Name |
Assays per Kit |
K - MBG4 |
Malt β - Glucanase/Lichenase Assay Kit |
100 |
K - MBGL |
β- Glucanase Assay Kit (Malt & Microbial) |
100 |
Xylanase | ||
Product Code |
Product Name |
Assays per Kit |
K - XylX6 - 2V |
endo - Xylanase Assay Kit |
200 |
Cellulase | ||
Product Code |
Product Name |
Assays per Kit |
K - CellG5 - 4V |
endo - Cellulase Assay Kit |
120 |

Analytical Solutions from Megazyme
▍▏Cultivating Excellence in Cereal Chemistry
Megazyme kits make enzymatic bio-analysis accessible for brewhouses and laboratories of any size. These assay kits offer a range of advantages, including:
• specific measurement of key analytes
• rapid analysis times
• simple formats
• long shelf life
Megazyme’s range of assays, substrates and enzymes makes it possible to quantify key parameters across all stages of beermaking using readily available glass-/ plastic-ware and a spectrophotometer.
▍▏Tailor-made Substrates
Our range of assay kits for enzyme activity are based around carefully designed synthetic substrates which are completely specific for the enzyme under evaluation.
The chemical structure of Megazyme’s substrates is unambiguous, unlike native polysaccharides. This means that the assay is highly reproducible and measurement of the target enzyme is specific.
▍▏Megazyme and Malt Analysis
Megazyme was recently involved in a project for the determination of six key enzyme activities in malts. Megazyme kits were used to measure the three starch hydrolases and the three main cell-wall hydrolases.
By carefully optimising extraction parameters, scientists at Megazyme were able to devise an extraction protocol that enables analysts to measure up to six key enzyme activities from a single malt extract.
What makes this protocol most appealing to breeders, maltsters and brewers is its full post-extraction auto matibility, meaning less hands-on time for analysts.

点在看,传递你的品味