【Megazyme】Assay Kits for Other Analytes(2)
List of Assay Kits
Analyte |
Product Code |
Reducing Sugars and Sucrose | |
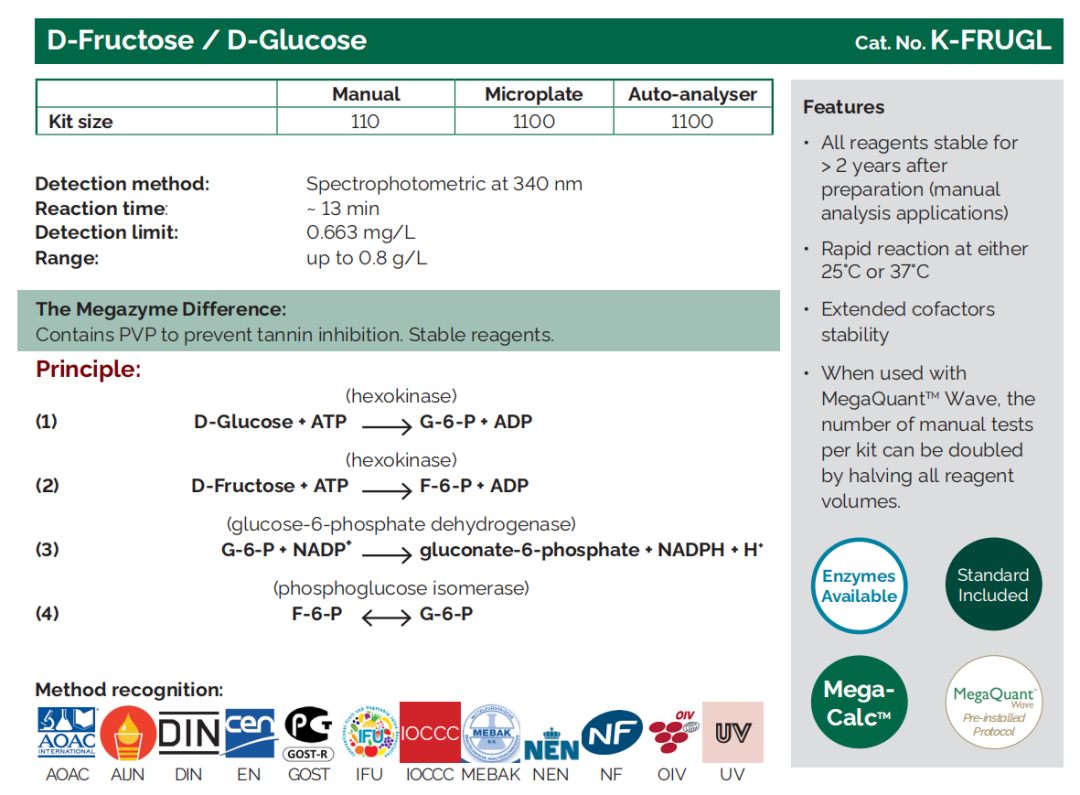
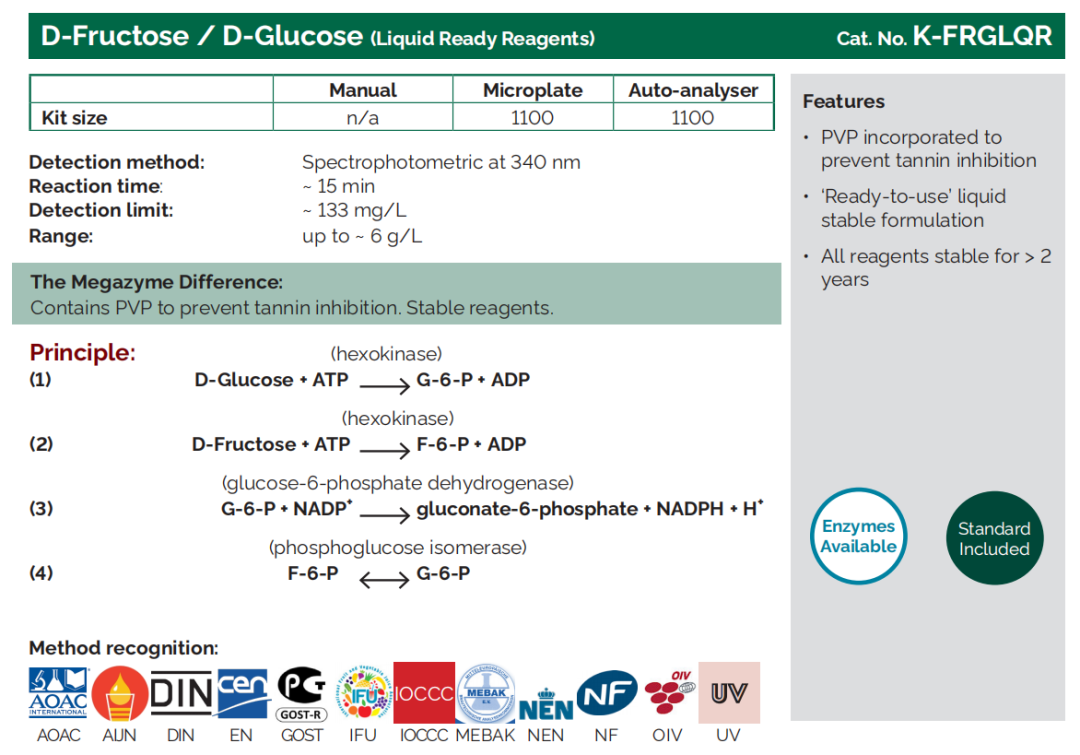
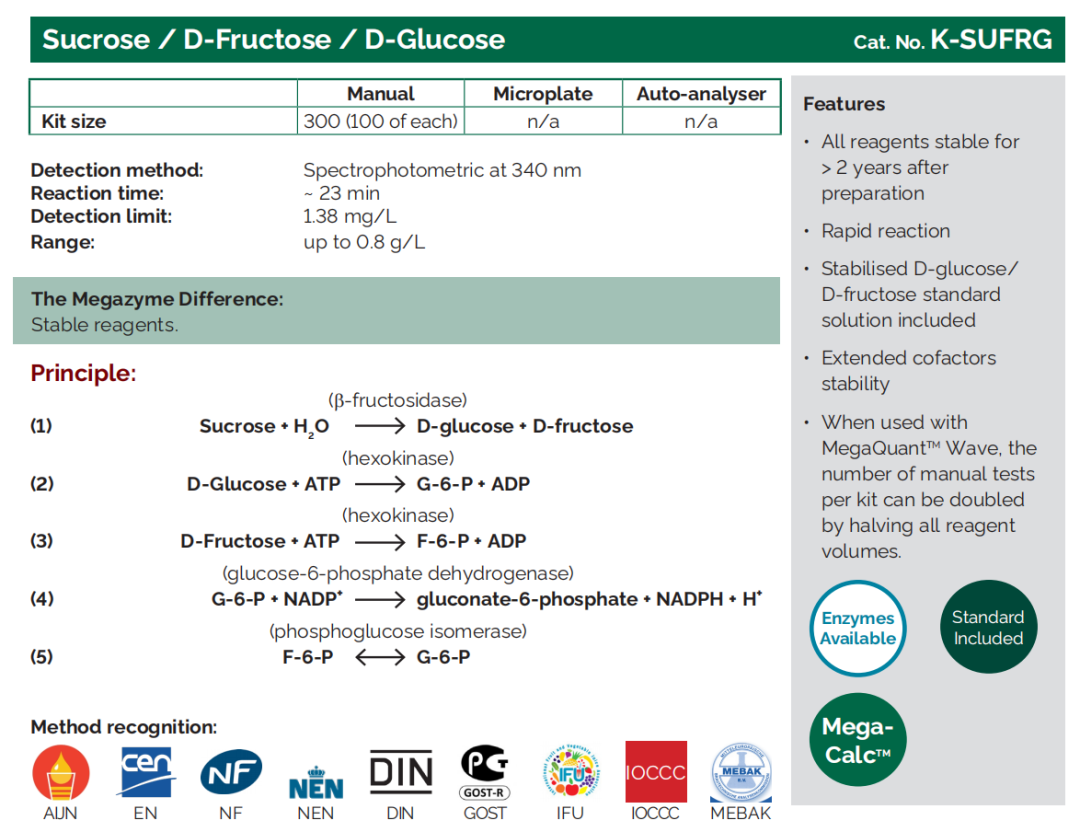
D - Fructose/D - Glucose |
K - FRUGL, K - FRGLQR |
Sucrose/D - Fructose/D - Glucose |
K - SUFRG |
Sulphites | |
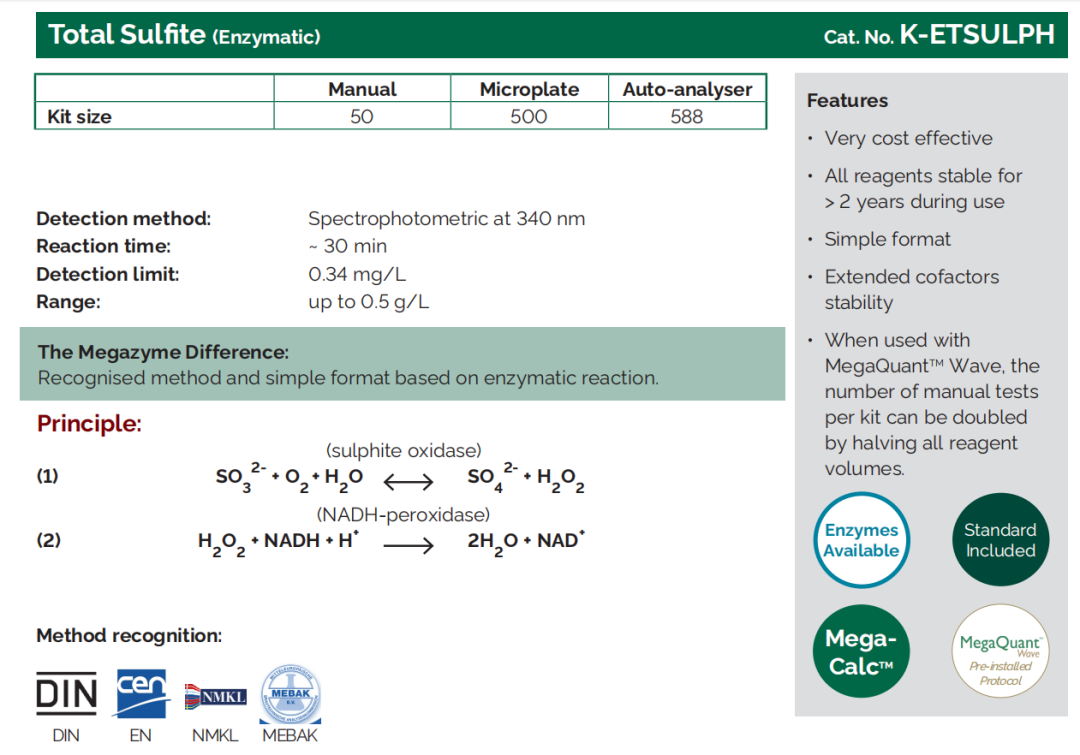
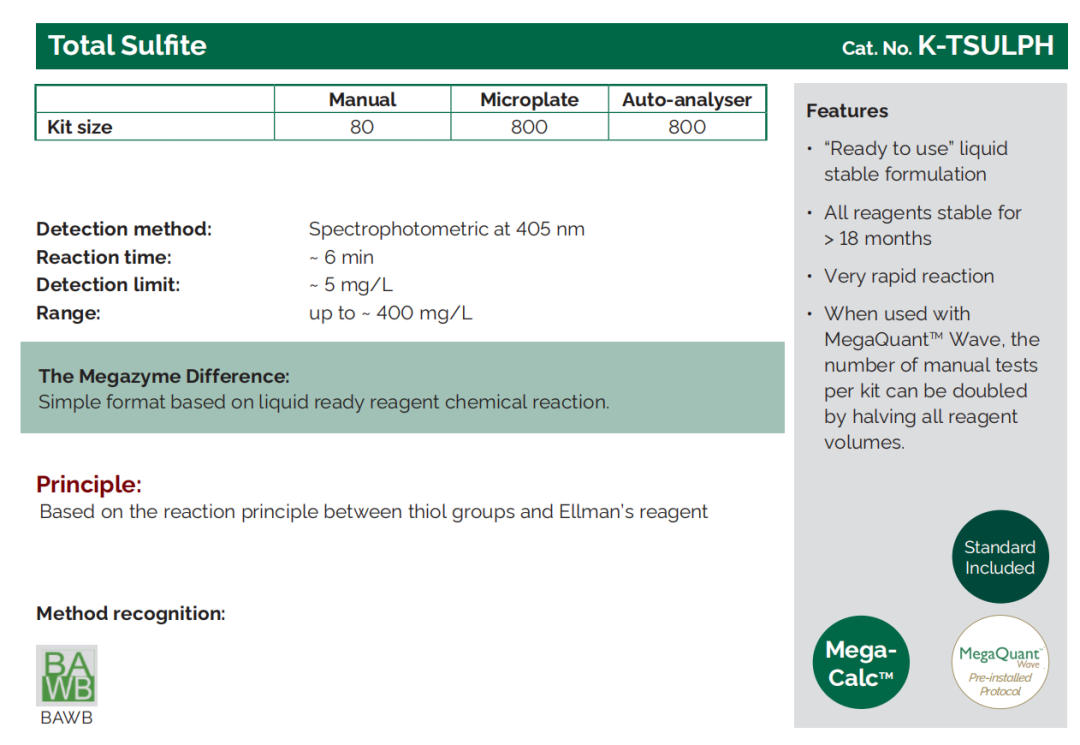
Total Sulphite |
K - ETSULPH, K - TSULPH |
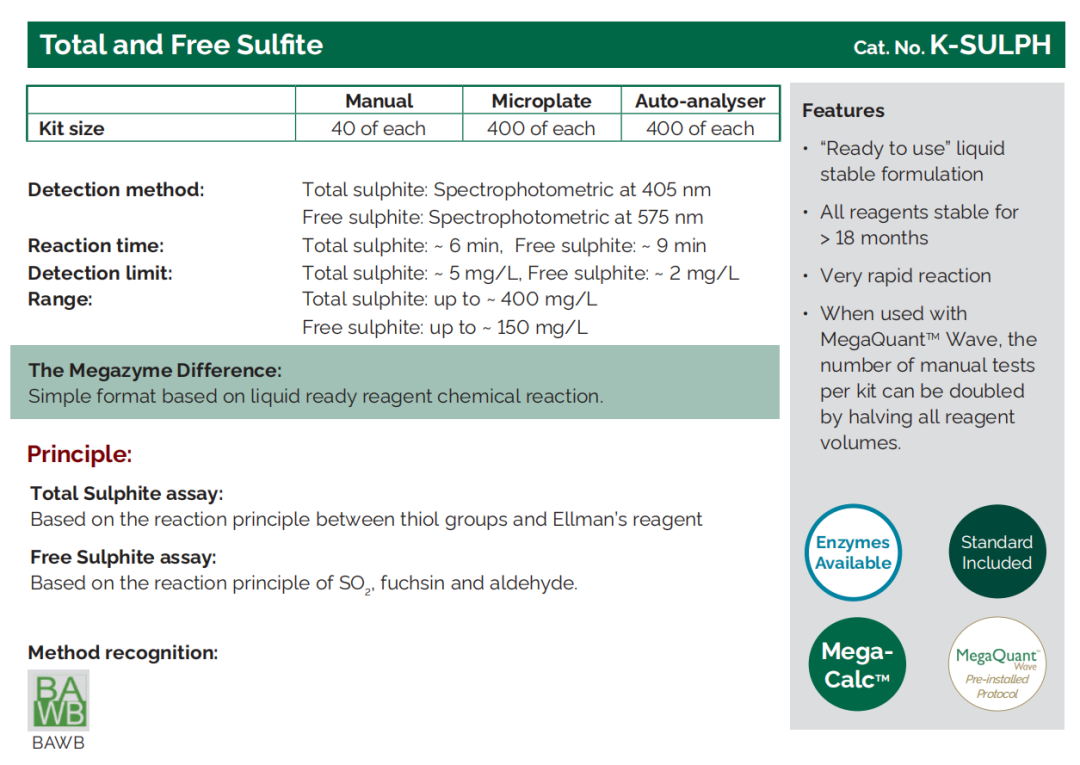
Total & Free Sulphite |
K - SULPH |
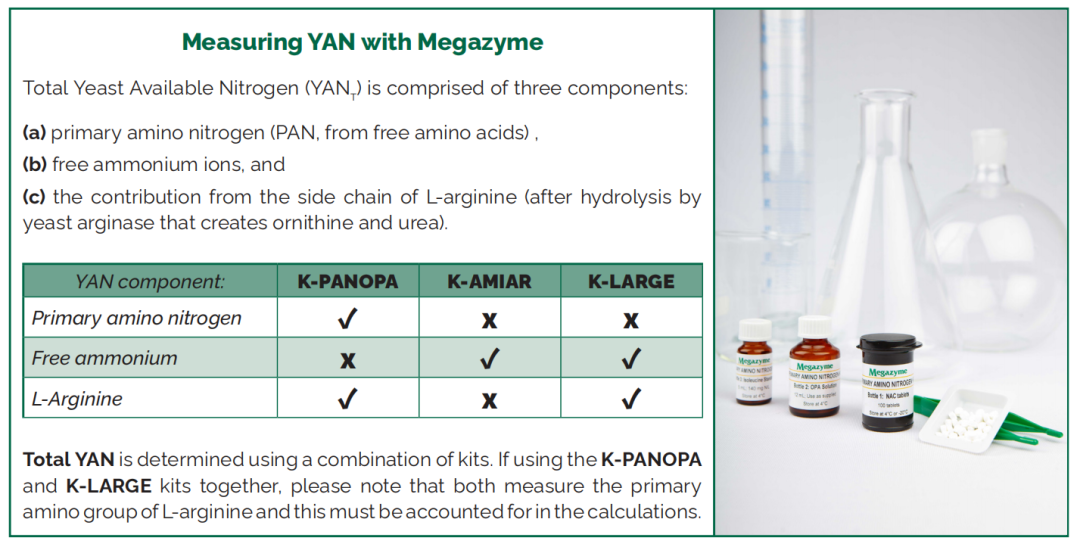
Yeast Assimilable Nitrogen (YAN) | |
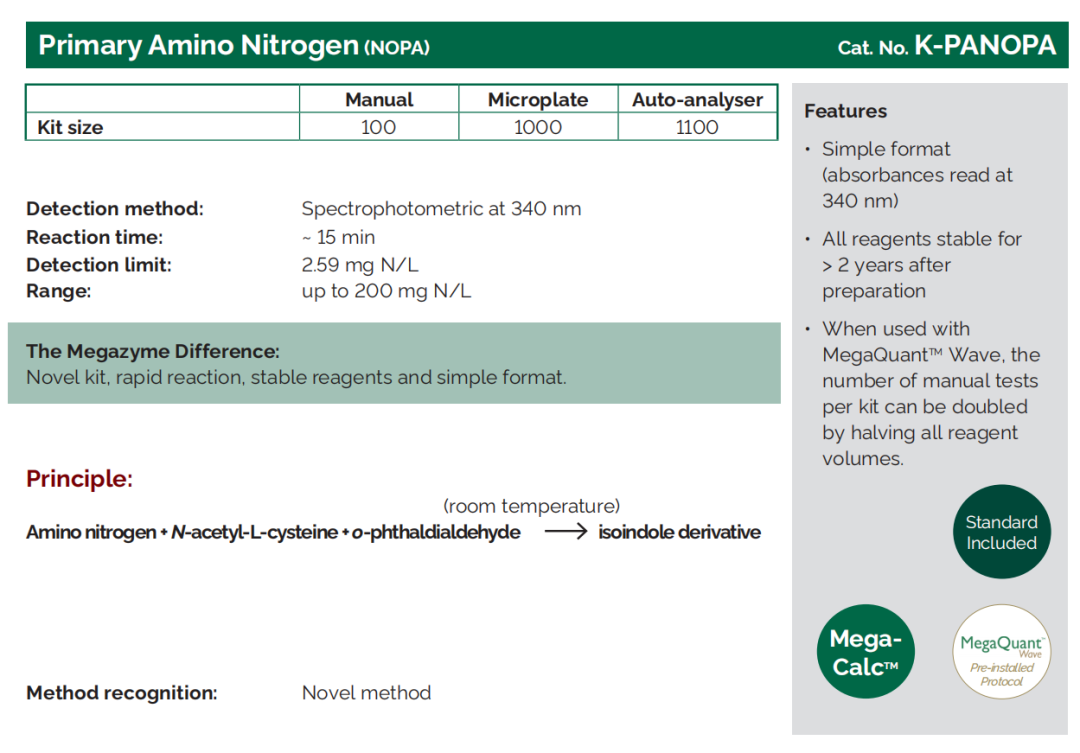
Primary Amino Nitrogen |
K - PANOPA |
L - Arginine/Urea /Ammonia (Rapid) |
K - LARGE |
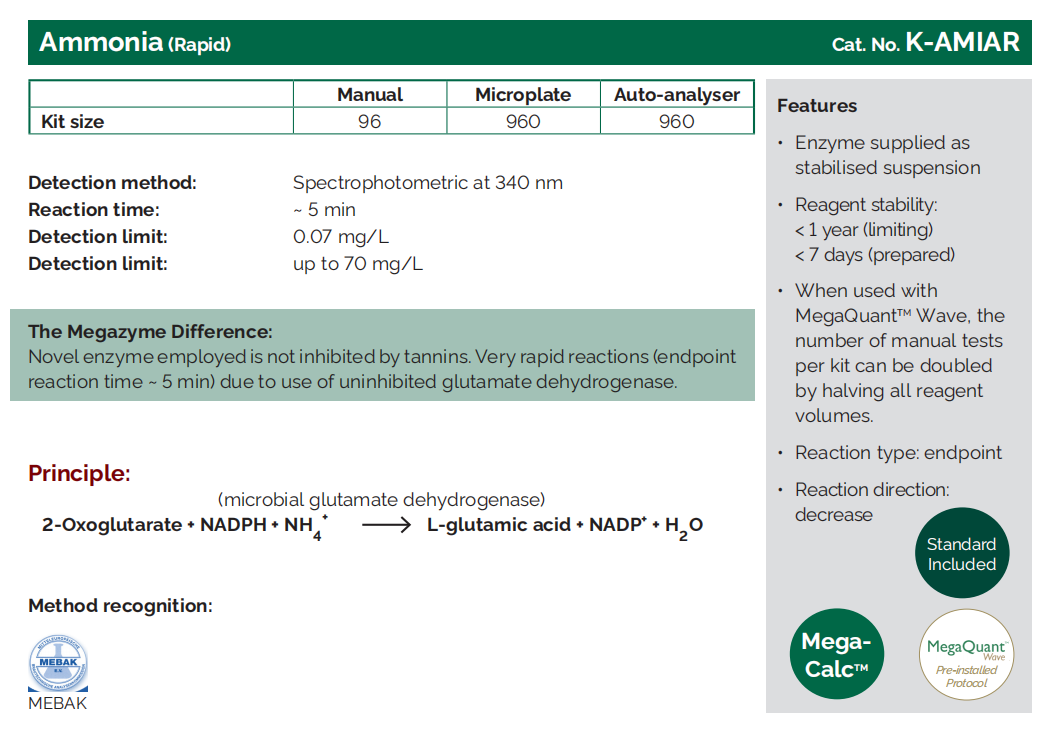
Ammonia (Rapid) |
K - AMIAR |
Guide to Symbols
The following icons, symbols and graphics are used in this catalogue.
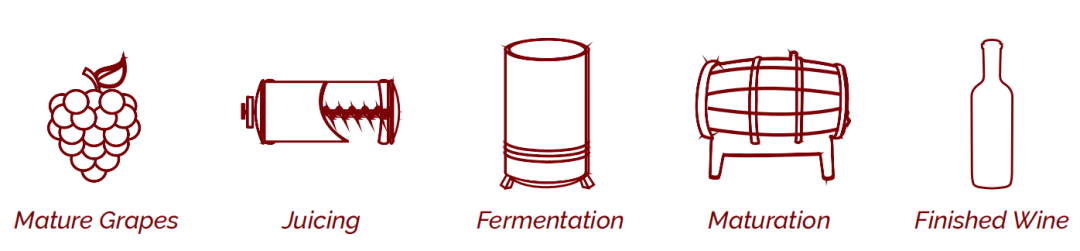
‘When to test’ graphic
Each analyte guide includes a graphic showing when that analyte is most commonly tested during the vinification process. The steps in the vinification process that are considered are as follows (also used on our wine infographic on page 5):
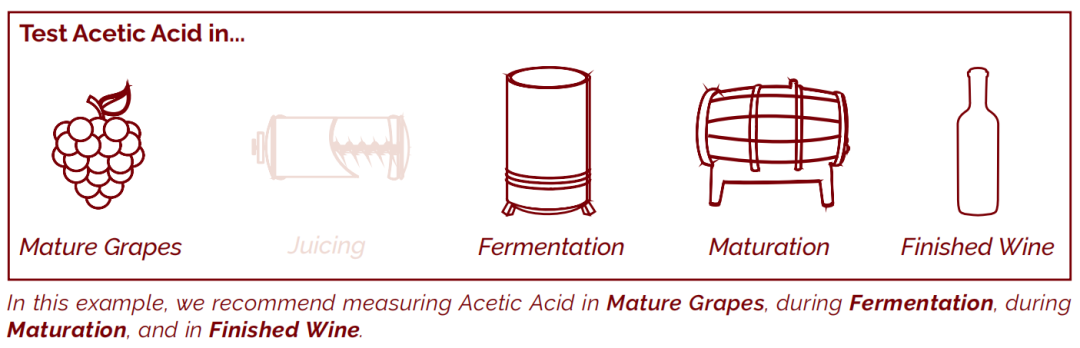
Every winery and wine will have its own unique testing requirements. However we have aimed to indicate the typical stages during winemaking when measurement of an analyte is likely to provide useful results that will direct further action. If we recommend testing at a specific step, it is displayed in a dark red colour and labelled as follows:
Icons for product features
The data listing for each Megazyme product is shown with a sidebar that summarises the product features. The following symbols are used where appropriate:

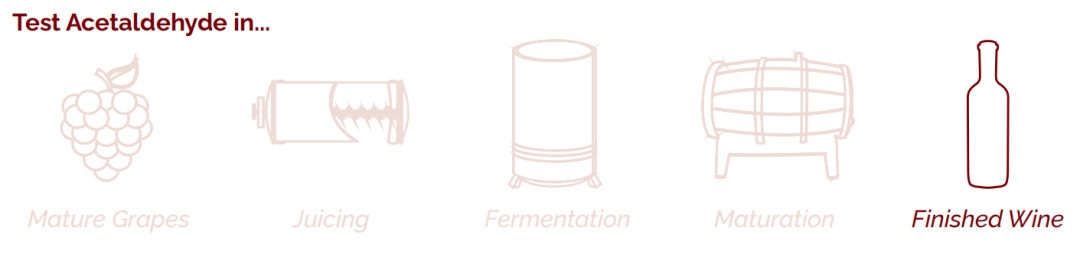
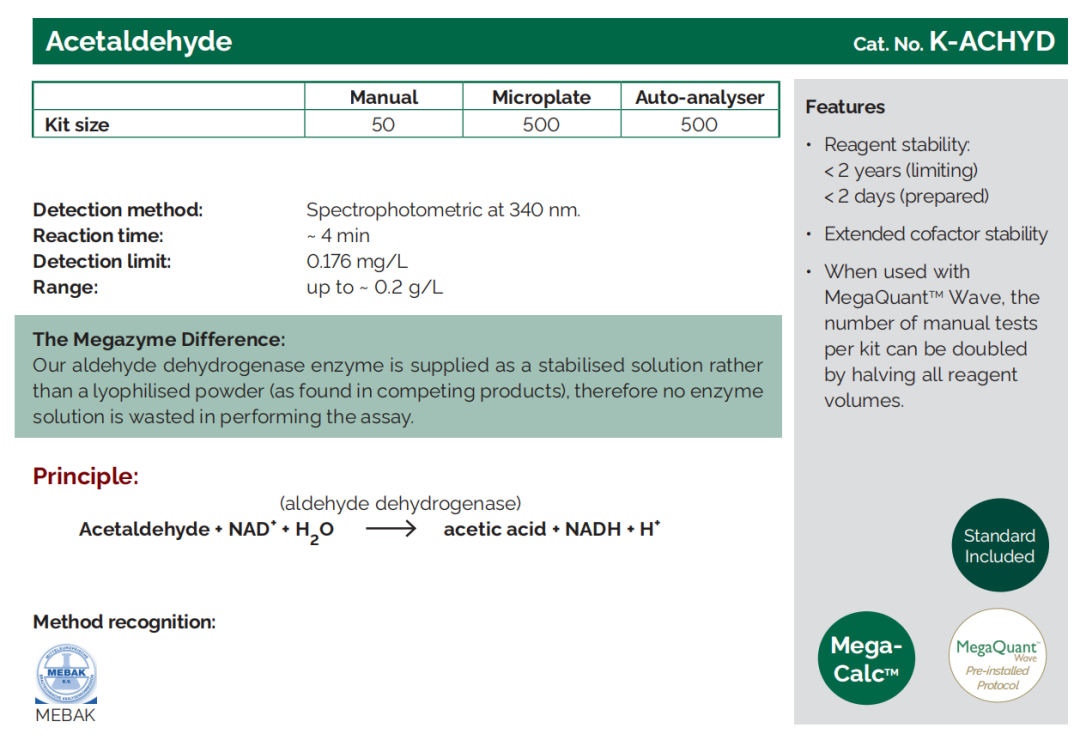
Acetaldehyde (sometimes called ethanal) is a sensory compound that adds flavour and complexity, but spoils wine at high concentrations.
Where does it come from?
Acetaldehyde can form at two different points in the vinification process. During yeast fermentation, by-products of ethanol formation are converted to acetaldehyde by the same micro-organisms responsible for producing acetic acid. Much of this acetaldehyde degrades during malolactic fermentation. However, during the maturation stage, ethanol in the wine may be oxidised to acetaldehyde by the enzyme alcohol dehydrogenase.
What does acetaldehyde mean for my wine?
At low levels (< 30 mg/L for red wine and < 80 mg/L for white wine), acetaldehyde imparts a pleasant “fruity” aroma to wine. Above this threshold, acetaldehyde can result in a metallic, “grassy”, sherry-like odour
What can I do with the acetaldehyde result?
High levels of acetaldehyde can be corrected by addition of sulphur dioxide (SO2 ). Sulphur dioxide and acetaldehyde bind together to form a compound called hydroxy-sulphonate, which does not influence the flavour and aroma of the wine.
Reducing Sugars (Glucose/Fructose) and Sucrose
Sugar influences alcohol content, flavour, and mouthfeel, and is measured throughout the production process.

Where does it come from?
Generated through photosynthesis, D-glucose and D-fructose occur naturally in grapes both as free sugars and the form of sucrose.
What does sugar mean for my wine?
The D-glucose and D-fructose content, often referred to as total reducing sugars, is considered a key quality parameter ahead of fermentation as it reflects the amount of sugar available to yeast for conversion into ethanol. By testing glucose and fructose in juice, winemakers can estimate the potential alcohol concentration of the finished wine.
The total glucose and fructose concentration that remains after fermentation - the residual sugar - indicates how “dry” the finished wine is likely to be.
The addition of sucrose is only permitted in a few situations, for example in the production of champagne. The practice of ‘chaptalisation’ involves adding sucrose to grape must in order to increase the amount of alcohol in the finished wine.
What can I do with the sugar result?
Monitoring of sugar levels at each stage of the winemaking process helps producers to make decisions that will influence the final composition and texture of the wine, for example around grape ripeness, whether to chaptalise, and when to stop fermentation.
Note: Sucrose is not a ‘reducing sugar’ but must be factored in when calculating a wine’s total sugar content.

Sulphites
Sulphites are used as an essential additive in the control of microbial contamination during aging and also to protect the wine against detrimental “oxidative and enzymatic browning”.
Where do they come from?
Sulphites occur naturally as a by-product of yeast fermentation. However, wine producers have also added sulphur dioxide (SO2 ) to wine for thousands of years due to its antimicrobial and antioxidant properties.
What do sulphites mean for my wine?
Sulphites enhance the microbial stability of a wine (discouraging the growth of spoilage bacteria) and interact with reactive molecules like acetaldehyde, which can cause oxidation if left untreated.
At concentrations of 100 mg/L, sulphites begin to affect taste, imparting a “burnt” flavour.
Most wines have SO2 added just before bottling in order to kill any remaining spoilage bacteria, and to bind to molecules in the wine that could affect its flavour as it ages. Some winemakers also find applications for SO2 at other points, for example to halt malolactic fermentation or to kill any wild yeasts present at the time of harvest/crushing.
SO2 is only active as an antimicrobial and antioxidant preservative in the unbound “free” form. Given that SO2 becomes “inactive” when it binds the colour pigments of wine, and with legal restrictions on SO2 levels in wine, it has become valuable to wine producers to measure both the Free SO2 (FSO2 ) and Total SO2 (TSO2 ).
What can I do with the sulphite result?
Any wine with a sulphur dioxide concentration over 10 mg/L is required to state on the label that it “contains sulphites.” It is not uncommon for wines to exceed this threshold even without the artificial addition of sulphur dioxide.
Winemakers test FSO2 to ensure that SO2 previously added has not entirely reacted away (indicating that more sulphur dioxide needs to be added).
TSO2 needs to be monitored to check how much SO2 has been added so far, in order to ensure that the wine remains compliant with local regulations.
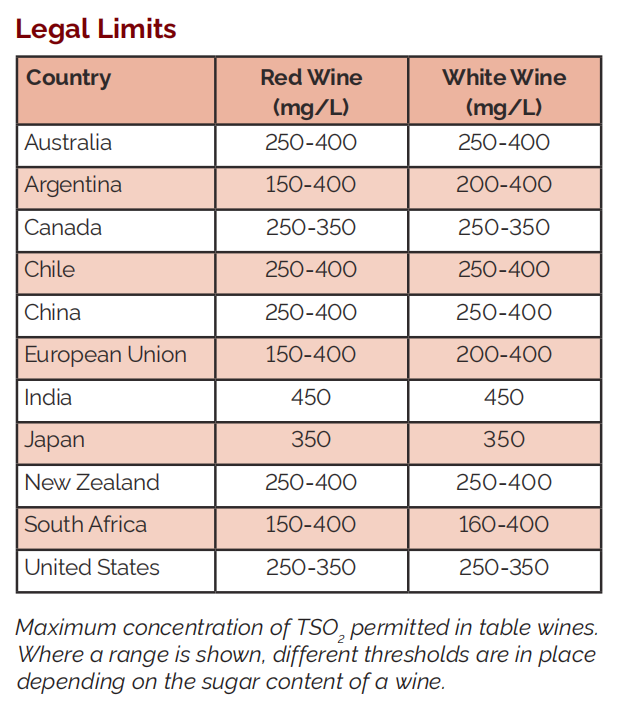
Many countries have set restrictions on the Total Sulphite concentration of wines made or sold within their jurisdiction. The limit may be different for dessert wines or other wines with a particularly high sugar content (typically defined as over 35 g/L). The table shows national regulations at the time of printing.
Yeast Assimilable Nitrogen
Nitrogen influences the activity of yeast before and during fermentation. Nitrogen-based components, e.g. proteins, also contribute to the mouthfeel of the finished wine.
Where does it come from?
Nitrogen is part of the amino acids and proteins found naturally in grapes and micro-organisms. Nitrogen compounds are important nutrients for yeast involved in fermentation. For this reason, the form of nitrogen most assayed in the wine industry is Yeast Assimilable Nitrogen (YAN), i.e. nitrogen available to yeast cells.
What does YAN mean for my wine?
Too little YAN can result in sluggish or “stuck” fermentation.
Too much nitrogen will mean there are nutrients available for spoilage organisms as well as yeast. Excess nitrogen can also lead to the formation of the carcinogenic compound ethyl carbamate (especially where starting levels of L-arginine in the juice are high).
What can I do with the result?
The result can be used to determine whether extra YAN should be added in the form of diammonium phosphate (DAP), and if so, how much can be added prudently.
Wines sold in the European Union may contain up to 1 g/L of ammonium salts.


点在看,传递你的品味